Alleviation of dry mouth by saliva substitutes improved swallowing ability and clinical nutritional status of post-radiotherapy head and neck cancer patients: a randomized controlled trial
- PMID: 31732852
- PMCID: PMC7181446
- DOI: 10.1007/s00520-019-05132-1
Alleviation of dry mouth by saliva substitutes improved swallowing ability and clinical nutritional status of post-radiotherapy head and neck cancer patients: a randomized controlled trial
Erratum in
-
Correction to: Alleviation of dry mouth by saliva substitutes improved swallowing ability and clinical nutritional status of post-radiotherapy head and neck cancer patients: a randomized controlled trial.Support Care Cancer. 2025 Sep 3;33(10):831. doi: 10.1007/s00520-025-09912-w. Support Care Cancer. 2025. PMID: 40897905 Free PMC article. No abstract available.
Abstract
Purpose: The aim of this study is to investigate the effect of an edible saliva substitute, oral moisturizing jelly (OMJ), and a topical saliva gel (GC) on dry mouth, swallowing ability, and nutritional status in post-radiotherapy head and neck cancer patients.
Methods: Sixty-two post-radiation head and neck cancer patients with xerostomia completed a blinded randomized controlled trial. They were advised to swallow OMJ (n = 31) or apply GC orally (n = 31) for 2 months. Outcome measures were assessed at baseline, 1, and 2 months, including subjective and objective dry mouth (Challcombe) scores, subjective swallowing problem scores (EAT-10), water swallowing time, clinical nutritional status (PG-SGA), body weight, and dietary intake.
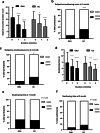
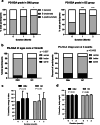
Results: After 1 and 2 months of interventions, subjective and objective dry mouth scores, subjective swallowing problem scores, swallowing times, and clinical nutritional status in both groups were significantly improved (p < 0.0001). Compared to GC, OMJ group had higher percent improvement in all outcome measures (p < 0.001) except swallowing time and clinical nutritional status. Interestingly, subjective dry mouth scores were significantly correlated with subjective swallowing problem scores (r = 0.5321, p < 0.0001).
Conclusions: Continuous uses of saliva substitutes (OMJ or GC) for at least a month improved signs and symptoms of dry mouth and enhanced swallowing ability. An edible saliva substitute was superior to a topical saliva gel for alleviating dry mouth and swallow problems. These lead to improved clinical nutritional status. Thus, palliation of dry mouth may be critical to support nutrition of post-radiotherapy head and neck cancer patients.
Clinical trial registry: Clinicaltrials.gov NCT03035825.
Keywords: Dysphagia; Head and neck cancer; Nutritional status; Radiation therapy; Saliva substitute; Xerostomia.
Conflict of interest statement
DT and AL received a grant support for this research from Dental Innovation Foundation under Royal Patronage, His Majesty the King Dental Service Unit. Other authors have no conflict of interest. The authors have full control of all primary data and agreed to allow the journal to review their data if requested.
Figures

References
-
- Guggenheimer J (2003) Xerostomia etiology, recognition and treatment. JADA 134:61–69 - PubMed
-
- Porter SR, Scully C, Hegarty AM (2004) An update of the etiology and management of xerostomia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 97:28–46 - PubMed
-
- Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP (2003) Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med 14:199–212 - PubMed
-
- Shiboski CH, Hodgson TA, Ship JA, Schidt M (2007) Management of salivary gland hypofunction during and after radiotherapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103:S66.e1–S66.e19 - PubMed
-
- Ramaekers BL, Joore MA, Grutters JP, van den Ende P, Jd J, Houben R et al (2011) The impact of late treatment-toxicity on generic health-related quality of life in head and neck cancer patients after radiotherapy. Oral Oncol 47:768–774 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

