Disparate Central and Peripheral Effects of Circulating IGF-1 Deficiency on Tissue Mitochondrial Function
- PMID: 31732912
- PMCID: PMC7060968
- DOI: 10.1007/s12035-019-01821-4
Disparate Central and Peripheral Effects of Circulating IGF-1 Deficiency on Tissue Mitochondrial Function
Abstract
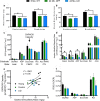
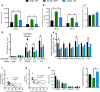
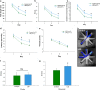
Age-related decline in circulating levels of insulin-like growth factor (IGF)-1 is associated with reduced cognitive function, neuronal aging, and neurodegeneration. Decreased mitochondrial function along with increased reactive oxygen species (ROS) and accumulation of damaged macromolecules are hallmarks of cellular aging. Based on numerous studies indicating pleiotropic effects of IGF-1 during aging, we compared the central and peripheral effects of circulating IGF-1 deficiency on tissue mitochondrial function using an inducible liver IGF-1 knockout (LID). Circulating levels of IGF-1 (~ 75%) were depleted in adult male Igf1f/f mice via AAV-mediated knockdown of hepatic IGF-1 at 5 months of age. Cognitive function was evaluated at 18 months using the radial arm water maze and glucose and insulin tolerance assessed. Mitochondrial function was analyzed in hippocampus, muscle, and visceral fat tissues using high-resolution respirometry O2K as well as redox status and oxidative stress in the cortex. Peripherally, IGF-1 deficiency did not significantly impact muscle mass or mitochondrial function. Aged LID mice were insulin resistant and exhibited ~ 60% less adipose tissue but increased fat mitochondrial respiration (20%). The effects on fat metabolism were attributed to increases in growth hormone. Centrally, IGF-1 deficiency impaired hippocampal-dependent spatial acquisition as well as reversal learning in male mice. Hippocampal mitochondrial OXPHOS coupling efficiency and cortex ATP levels (~ 50%) were decreased and hippocampal oxidative stress (protein carbonylation and F2-isoprostanes) was increased. These data suggest that IGF-1 is critical for regulating mitochondrial function, redox status, and spatial learning in the central nervous system but has limited impact on peripheral (liver and muscle) metabolism with age. Therefore, IGF-1 deficiency with age may increase sensitivity to damage in the brain and propensity for cognitive deficits. Targeting mitochondrial function in the brain may be an avenue for therapy of age-related impairment of cognitive function. Regulation of mitochondrial function and redox status by IGF-1 is essential to maintain brain function and coordinate hippocampal-dependent spatial learning. While a decline in IGF-1 in the periphery may be beneficial to avert cancer progression, diminished central IGF-1 signaling may mediate, in part, age-related cognitive dysfunction and cognitive pathologies potentially by decreasing mitochondrial function.
Keywords: Cognitive function; IGF-1; Learning and memory; Mitochondria; Oxidative stress; ROS.
Figures


Similar articles
-
Insulin-like growth factor receptor signaling regulates working memory, mitochondrial metabolism, and amyloid-β uptake in astrocytes.Mol Metab. 2018 Mar;9:141-155. doi: 10.1016/j.molmet.2018.01.013. Epub 2018 Feb 2. Mol Metab. 2018. PMID: 29398615 Free PMC article.
-
Long-term deficiency of circulating and hippocampal insulin-like growth factor I induces depressive behavior in adult mice: a potential model of geriatric depression.Neuroscience. 2011 Jun 30;185:50-60. doi: 10.1016/j.neuroscience.2011.04.032. Epub 2011 Apr 20. Neuroscience. 2011. PMID: 21524689 Free PMC article.
-
Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging.Age (Dordr). 2016 Aug;38(4):273-289. doi: 10.1007/s11357-016-9931-0. Epub 2016 Sep 9. Age (Dordr). 2016. PMID: 27613724 Free PMC article.
-
Insulin-like Growth Factor 1 (IGF-1) as a marker of cognitive decline in normal ageing: A review.Ageing Res Rev. 2018 Mar;42:14-27. doi: 10.1016/j.arr.2017.12.002. Epub 2017 Dec 9. Ageing Res Rev. 2018. PMID: 29233786
-
Insulin-like growth factor-1 and cognitive health: Exploring cellular, preclinical, and clinical dimensions.Front Neuroendocrinol. 2025 Jan;76:101161. doi: 10.1016/j.yfrne.2024.101161. Epub 2024 Nov 12. Front Neuroendocrinol. 2025. PMID: 39536910 Review.
Cited by
-
TREM2-IGF1 Mediated Glucometabolic Enhancement Underlies Microglial Neuroprotective Properties During Ischemic Stroke.Adv Sci (Weinh). 2024 Mar;11(10):e2305614. doi: 10.1002/advs.202305614. Epub 2023 Dec 27. Adv Sci (Weinh). 2024. PMID: 38151703 Free PMC article.
-
A Balanced Act: The Effects of GH-GHR-IGF1 Axis on Mitochondrial Function.Front Cell Dev Biol. 2021 Mar 18;9:630248. doi: 10.3389/fcell.2021.630248. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33816476 Free PMC article. Review.
-
The Role of Insulin-like Growth Factor I in Mechanisms of Resilience and Vulnerability to Sporadic Alzheimer's Disease.Int J Mol Sci. 2023 Nov 17;24(22):16440. doi: 10.3390/ijms242216440. Int J Mol Sci. 2023. PMID: 38003628 Free PMC article. Review.
-
Nanotechnology-Based Drug Delivery Strategies to Repair the Mitochondrial Function in Neuroinflammatory and Neurodegenerative Diseases.Pharmaceutics. 2021 Dec 1;13(12):2055. doi: 10.3390/pharmaceutics13122055. Pharmaceutics. 2021. PMID: 34959337 Free PMC article. Review.
-
Early Life Interventions: Impact on Aging and Longevity.Aging Dis. 2024 Jul 5;16(5):2659-2673. doi: 10.14336/AD.202.0516. Aging Dis. 2024. PMID: 39325935 Free PMC article. Review.
References
-
- Poon HF, Calabrese V, Scapagnini G, Butterfield DA. Free radicals and brain aging. Clin Geriatr Med. 2004;20(2):329–359. - PubMed
-
- Poon HF, et al. Free radicals: key to brain aging and heme oxygenase as a cellular response to oxidative stress. J Gerontol A Biol Sci Med Sci. 2004;59(5):478–493. - PubMed
-
- Parihar MS, Brewer GJ. Simultaneous age-related depolarization of mitochondrial membrane potential and increased mitochondrial reactive oxygen species production correlate with age-related glutamate excitotoxicity in rat hippocampal neurons. J Neurosci Res. 2007;85(5):1018–1032. - PubMed
-
- Parihar MS, Kunz EA, Brewer GJ. Age-related decreases in NAD(P)H and glutathione cause redox declines before ATP loss during glutamate treatment of hippocampal neurons. J Neurosci Res. 2008;86(10):2339–2352. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

