Construct validity of the Physiotherapy Evidence Database (PEDro) quality scale for randomized trials: Item response theory and factor analyses
- PMID: 31733091
- PMCID: PMC7079093
- DOI: 10.1002/jrsm.1385
Construct validity of the Physiotherapy Evidence Database (PEDro) quality scale for randomized trials: Item response theory and factor analyses
Abstract
Background: There is an agreement that the methodological quality of randomized trials should be assessed in systematic reviews, but there is a debate on how this should be done. We conducted a construct validation study of the Physiotherapy Evidence Database (PEDro) scale, which is widely used to assess the quality of trials in physical therapy and rehabilitation.
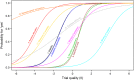
Methods: We analyzed 345 trials that were included in Cochrane reviews and for which a PEDro summary score was available. We used one- and two-parameter logistic item response theory (IRT) models to study the psychometric properties of the PEDro scale and assessed the items' difficulty and discrimination parameters. We ran goodness of fit post estimations and examined the IRT unidimensionality assumption with a multidimensional IRT (MIRT) model.
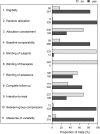
Results: Out of a maximum of 10, the mean PEDro summary score was 5.46 (SD = 1.51). The allocation concealment and intention-to-treat scale items contributed most of the information on the underlying construct (with discriminations of 1.79 and 2.05, respectively) at similar difficulties (0.63 and 0.65, respectively). The other items provided little additional information and did not distinguish trials of different quality. There was substantial evidence of departure from the unidimensionality assumption, suggesting that the PEDro items relate to more than one latent trait.
Conclusions: Our findings question the construct validity of the PEDro scale to assess the methodological quality of clinical trials. PEDro summary scores should not be used; rather, the physiotherapy community should consider working with the individual items of the scale.
Keywords: item response theory; physiotherapy; randomized clinical trials; risk of bias; study quality scale; validation.
© 2019 The Authors. Research Synthesis Methods published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors reported no conflict of interest.
Figures

References
-
- Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: synthesis of best evidence for clinical decisions. Ann Intern Med. 1997;126(5):376‐380. - PubMed
-
- Jüni P, Witschi A, Bloch R, Egger M, Juni P. The hazards of scoring the quality of clinical trials for meta‐analysis. JAMA J Am Med Assoc. 1999;282(11):1054‐1060. - PubMed
-
- Moher D, Jadad AR, Nichol G, Penman M, Tugwell P, Walsh S. Assessing the quality of randomized controlled trials: an annotated bibliography of scales and checklists. Control Clin Trials. 1995;16(1):62‐73. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
