Intracardiac administration of ephrinA1-Fc preserves mitochondrial bioenergetics during acute ischemia/reperfusion injury
- PMID: 31733316
- PMCID: PMC6898793
- DOI: 10.1016/j.lfs.2019.117053
Intracardiac administration of ephrinA1-Fc preserves mitochondrial bioenergetics during acute ischemia/reperfusion injury
Abstract
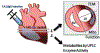
Aims: Intracardiac injection of recombinant EphrinA1-Fc immediately following coronary artery ligation in mice reduces infarct size in both reperfused and non-reperfused myocardium, but the cellular alterations behind this phenomenon remain unknown.
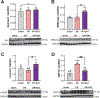
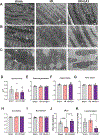
Main methods: Herein, 10 wk-old B6129SF2/J male mice were exposed to acute ischemia/reperfusion (30minI/24hrsR) injury immediately followed by intracardiac injection of either EphrinA1-Fc or IgG-Fc. After 24 h of reperfusion, sections of the infarct margin in the left ventricle were imaged via transmission electron microscopy, and mitochondrial function was assessed in both permeabilized fibers and isolated mitochondria, to examine mitochondrial structure, function, and energetics in the early stages of repair.
Key findings: At a structural level, EphrinA1-Fc administration prevented the I/R-induced loss of sarcomere alignment and mitochondrial organization along the Z disks, as well as disorganization of the cristae and loss of inter-mitochondrial junctions. With respect to bioenergetics, loss of respiratory function induced by I/R was prevented by EphrinA1-Fc. Preservation of cardiac bioenergetics was not due to changes in mitochondrial JH2O2 emitting potential, membrane potential, ADP affinity, efficiency of ATP production, or activity of the main dehydrogenase enzymes, suggesting that EphrinA1-Fc indirectly maintains respiratory function via preservation of the mitochondrial network. Moreover, these protective effects were lost in isolated mitochondria, further emphasizing the importance of the intact cardiomyocyte ultrastructure in mitochondrial energetics.
Significance: Collectively, these data suggest that intracardiac injection of EphrinA1-Fc protects cardiac function by preserving cardiomyocyte structure and mitochondrial bioenergetics, thus emerging as a potential therapeutic strategy in I/R injury.
Keywords: Cardioprotection; Mitochondrial bioenergetics; Myocardial infarction; ephrinA1.
Published by Elsevier Inc.
Conflict of interest statement
Conflict of Interest
A conflicting interest exists when professional judgment concerning a primary interest (such as patient’s welfare or the validity of research) may be influenced by a secondary interest (such as financial gain or personal rivalry). It may arise for the authors when they have financial interest that may influence their interpretation of their results or those of others. Examples of potential conflicts of interest include employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding.
Figures
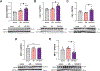

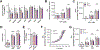


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

