Convergent extension in mammalian morphogenesis
- PMID: 31734039
- PMCID: PMC7071967
- DOI: 10.1016/j.semcdb.2019.11.002
Convergent extension in mammalian morphogenesis
Abstract
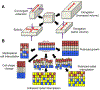
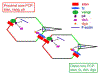
Convergent extension is a fundamental morphogenetic process that underlies not only the generation of the elongated vertebrate body plan from the initially radially symmetrical embryo, but also the specific shape changes characteristic of many individual tissues. These tissue shape changes are the result of specific cell behaviors, coordinated in time and space, and affected by the physical properties of the tissue. While mediolateral cell intercalation is the classic cellular mechanism for producing tissue convergence and extension, other cell behaviors can also provide similar tissue-scale distortions or can modulate the effects of mediolateral cell intercalation to sculpt a specific shape. Regulation of regional tissue morphogenesis through planar polarization of the variety of underlying cell behaviors is well-recognized, but as yet is not well understood at the molecular level. Here, we review recent advances in understanding the cellular basis for convergence and extension and its regulation.
Keywords: Axial elongation; Convergent extension; Gastrulation; Morphogenesis; Mouse; Planar cell polarity.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures



References
-
- Condic ML, Fristrom D, Fristrom JW, Apical cell shape changes during Drosophila imaginal leg disc elongation: A novel morphogenetic mechanism, Development. 111 (1991) 23–33. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

