Evaluation of the efficacy of Lactobacillus plantarum HEAL9 and Lactobacillus paracasei 8700:2 on aspects of common cold infections in children attending day care: a randomised, double-blind, placebo-controlled clinical study
- PMID: 31734734
- PMCID: PMC7000506
- DOI: 10.1007/s00394-019-02137-8
Evaluation of the efficacy of Lactobacillus plantarum HEAL9 and Lactobacillus paracasei 8700:2 on aspects of common cold infections in children attending day care: a randomised, double-blind, placebo-controlled clinical study
Erratum in
-
Correction to: Evaluation of the efficacy of Lactobacillus plantarum HEAL9 and Lactobacillus paracasei 8700:2 on aspects of common cold infections in children attending day care: a randomised, double-blind, placebo-controlled clinical study.Eur J Nutr. 2020 Feb;59(1):419. doi: 10.1007/s00394-019-02156-5. Eur J Nutr. 2020. PMID: 31858212 Free PMC article.
Abstract
Background: The combination of Lactobacillus plantarum HEAL9 and Lactobacillus paracasei 8700:2 (commercially available as Probi Defendum®) has previously been reported to reduce the incidence, duration and severity of naturally acquired common colds in adults. The aim of the present study was to evaluate the impact of Probi Defendum® on aspects of common cold in healthy children 1-6 years of age attending day care.
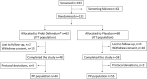
Methods: A total of 131 children, out of the planned 320, were recruited into the study during 1 common cold season and randomised to consume once daily either 109 CFU (colony forming units) of the probiotic product or placebo. Due to unforeseen reasons, the recruitment of more children did not continue beyond the first cold season.
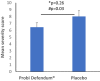
Results: There were 106 children that completed the study out of the 131 randomised. Daily consumption of the probiotic product for a period of 3 months significantly reduced the severity of the symptom "nasal congestion/runny nose" with a mean severity score for the whole study period of 7.5 ± 9.7 in the probiotic group and 13.9 ± 15.2 in the placebo (p < 0.05). Moreover, significantly less concomitant medication was used in the probiotic group. When the data were projected to a larger population corresponding to the originally estimated sample size, the results were in favour of the probiotic group regarding the reduced absence from day care (p < 0.05), reduced mean total severity per day in the reported episodes (p < 0.05) and reduced severity of the symptom "crying more than usual" (p < 0.05).
Conclusion: Intake of Probi Defendum® once daily for a period of 3 months was beneficial to children and reduced the severity of common colds.
Keywords: Common cold; Lactobacillus paracasei; Lactobacillus plantarum; Probi Defendum®; Probiotic; Respiratory tract infections.
Conflict of interest statement
Probi AB is the sponsor of this clinical study and the authors of the manuscript are employed by Probi AB or were employed at the time the study was initiated. The authors have been involved in the design of the study and the drafting of the manuscript but a CRO has been responsible for the clinical phase, data management and statistical analysis of data. Study conception and design were performed by Anna Berggren, Niklas Larsson and Irini Lazou Ahrén, the first draft of the manuscript was written by Irini Lazou Ahrén and all authors reviewed and commented on previous versions of the manuscript.
Figures
References
-
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. - DOI - PubMed
-
- King S, Glanville J, Sanders ME, Fitzgerald A, Varley D. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112(1):41–54. doi: 10.1017/S0007114514000075. - DOI - PMC - PubMed
-
- Wang Y, Li X, Ge T, Xiao Y, Liao Y, Cui Y, Zhang Y, Ho W, Yu G, Zhang T. Probiotics for prevention and treatment of respiratory tract infections in children: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2016;95(31):e4509. doi: 10.1097/MD.0000000000004509. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical