Effects of Venous Angioplasty on Cerebral Lesions in Multiple Sclerosis: Expanded Analysis of the Brave Dreams Double-Blind, Sham-Controlled Randomized Trial
- PMID: 31735108
- PMCID: PMC6970429
- DOI: 10.1177/1526602819890110
Effects of Venous Angioplasty on Cerebral Lesions in Multiple Sclerosis: Expanded Analysis of the Brave Dreams Double-Blind, Sham-Controlled Randomized Trial
Erratum in
-
Corrigendum.J Endovasc Ther. 2020 Feb;27(1):NP1. doi: 10.1177/1526602819898319. Epub 2020 Jan 2. J Endovasc Ther. 2020. PMID: 31896294 Free PMC article. No abstract available.
Abstract
Purpose: To evaluate if jugular vein flow restoration in various venographic defects indicative of chronic cerebrospinal venous insufficiency (CCSVI) in multiple sclerosis (MS) patients can have positive effects on cerebral lesions identified using magnetic resonance imaging (MRI).
Materials and methods: The Brave Dreams trial ( ClinicalTrials.gov identifier NCT01371760) was a multicenter, randomized, parallel group, double-blind, sham-controlled trial to assess the efficacy of jugular venoplasty in MS patients with CCSVI. Between August 2012 and March 2016, 130 patients (mean age 39.9±10.6 years; 81 women) with relapsing/remitting (n=115) or secondary/progressive (n=15) MS were randomized 2:1 to venography plus angioplasty (n=86) or venography (sham; n=44). Patients and study personnel (except the interventionist) were masked to treatment assignment. MRI data acquired at 6 and 12 months after randomization were compared to the preoperative scan for new and/or >30% enlargement of T2 lesions plus new gadolinium enhancement of pre-existing lesions. The relative risks (RR) with 95% confidence interval (CI) were estimated and compared. In a secondary assessment, venograms of patients who underwent venous angioplasty were graded as "favorable" (n=38) or "unfavorable" (n=30) for dilation according to the Giaquinta grading system by 4 investigators blinded to outcomes. These subgroups were also compared.
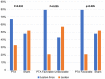
Results: Of the 130 patients enrolled, 125 (96%) completed the 12-month MRI follow-up. Analysis showed that the likelihood of being free of new cerebral lesions at 1 year was significantly higher after venoplasty compared to the sham group (RR 1.42, 95% CI 1.00 to 2.01, p=0.032). Patients with favorable venograms had a significantly higher probability of being free of new cerebral lesions than patients with unfavorable venograms (RR 1.82, 95% CI 1.17 to 2.83, p=0.005) or patients in the sham arm (RR 1.66, 95% CI 1.16 to 2.37, p=0.005).
Conclusion: Expanded analysis of the Brave Dreams data that included secondary/progressive MS patients in addition to the relapsing/remitting patients analyzed previously showed that venoplasty decreases new cerebral lesions at 1 year. Secondary analysis confirmed the efficacy of the Giaquinta grading system in selecting patients appropriate for venoplasty who were more likely to be free from accumulation of new cerebral lesions at MRI.
Keywords: angioplasty; cerebral drainage; cerebral lesion; chronic cerebrospinal venous insufficiency; echo Doppler; internal jugular vein; jugular flow; magnetic resonance imaging; multiple sclerosis; stenosis; vein defects; venography; venoplasty.
Conflict of interest statement
Figures


Comment in
-
Commentary: "Brave Dreams" Reanalysis Sheds New Light on Angioplasty for Venous Anomalies in Some Multiple Sclerosis Patients With Chronic Cerebrospinal Venous Insufficiency.J Endovasc Ther. 2020 Feb;27(1):18-19. doi: 10.1177/1526602819894300. J Endovasc Ther. 2020. PMID: 31948374 No abstract available.
References
-
- Lee BB, Baumgartner I, Berlien P, et al.; International Union of Phlebology. Diagnosis and treatment of venous malformations. Consensus document of the International Union of Phlebology (IUP): updated 2013. Int Angiol. 2015;34:97–149. - PubMed
-
- Pedriali M, Zamboni P. The pathology of the internal jugular vein wall in multiple sclerosis. J Mult Scler (Foster City). 2015;2:160. doi:10.4172/2376-0389.1000160 - DOI
-
- Zivadinov R, Bastianello S, Dake MD, et al.; International Society for Neurovascular Disease. Recommendations for multimodal noninvasive and invasive screening for detection of extracranial venous abnormalities indicative of chronic cerebrospinal venous insufficiency: a position statement of the International Society for Neurovascular Disease. J Vasc Interv Radiol. 2014;25:1785–1794.e17. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

