H19 promote calcium oxalate nephrocalcinosis-induced renal tubular epithelial cell injury via a ceRNA pathway
- PMID: 31735555
- PMCID: PMC6921206
- DOI: 10.1016/j.ebiom.2019.10.059
H19 promote calcium oxalate nephrocalcinosis-induced renal tubular epithelial cell injury via a ceRNA pathway
Abstract
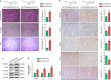
Background: Intrarenal calcium oxalate (CaOx) crystals induce inflammation and kidney tubular cell injury, which are processes that involve TLR4/NF-κB signalling. A recent genome-wide gene expression profile analysis of Randall's plaques in CaOx stone patients revealed that the expression of the long noncoding RNA H19 was significantly upregulated. However, to date, its role in kidney CaOx stones has not been reported.
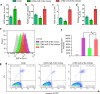
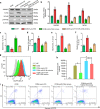
Method: A Gene Expression Omnibus (GEO) dataset was utilized to analyse gene expression profiles. Luciferase reporter, Western blotting, qRT-PCR, immunofluorescence staining and reactive oxygen species (ROS) assays were employed to study the molecular mechanism of HMGB1/TLR4/NF-κB regulation by H19 and miR-216b. In vitro and in vivo assays were performed to further confirm the proinflammatory and prooxidative stress effects.
Finding: H19 expression was significantly increased and positively correlated with the expression levels of HMGB1, TLR4 and NF-κB in Randall's plaques and glyoxylate-induced CaOx nephrocalcinosis mouse models. H19 interacted with miR-216b and suppressed its expression. Additionally, miR-216b inhibited HMGB1 expression by directly binding its 3'-untranslated region. Moreover, H19 downregulation inhibited HMGB1, TLR4 and NF-κB expression and suppressed CaOx nephrocalcinosis-induced renal tubular epithelial cell injury, NADPH oxidase, and oxidative stress in vivo and in vitro. Interestingly, miR-216b inhibition partially reversed the inhibitory effect of H19 knockdown on HMGB1 expression.
Interpretation: We determined that H19 might serve as a facilitator in the process of CaOx nephrocalcinosis-induced oxidative stress and renal tubular epithelial cell injury, and we revealed that the interaction between H19 and miR-216b could exert its effect via the HMGB1/TLR4/NF-κB pathway.
Funding: This work was supported by the National Nature Science Foundation of China (Nos. 8196030190, 8190033175, 81370805, 81470935, 81900645, 81500534, and 81602236).
Keywords: H19; HMGB1; calcium oxalate; ceRNA; tubular epithelial cell injury.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors have nothing to disclose.
Figures






References
-
- Zeng G., Mai Z., Xia S. Prevalence of kidney stones in China: an ultrasonography based cross-sectional study. BJU Int. 2017;120(1):109–116. - PubMed
-
- Ruan Y., Wang L., Zhao Y. Carbon monoxide potently prevents ischemia-induced high-mobility group box 1 translocation and release and protects against lethal renal ischemia-reperfusion injury. Kidney Int. 2014;86(3):525–537. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

