Insights into the interaction of Scedosporium apiospermum, Scedosporium aurantiacum, Scedosporium minutisporum, and Lomentospora prolificans with lung epithelial cells
- PMID: 31736016
- PMCID: PMC7203299
- DOI: 10.1007/s42770-019-00183-2
Insights into the interaction of Scedosporium apiospermum, Scedosporium aurantiacum, Scedosporium minutisporum, and Lomentospora prolificans with lung epithelial cells
Abstract
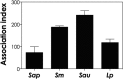
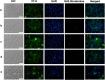
Scedosporium spp. and Lomentospora prolificans are filamentous fungi that emerged as human pathogens; however, their mechanisms of virulence/pathogenesis are still largely unknown. In the present work, we have evaluated the interaction of S. apiospermum, S. minutisporum, S. aurantiacum, and L. prolificans with lung epithelial cells (A549 line). The results showed that conidia were able to interact with A549 cells, displaying association indexes of 73.20, 117.98, 188.01, and 241.63 regarding S. apiospermum, L. prolificans, S. minutisporum, and S. aurantiacum, respectively. Light microscopy images evidenced morphological changes in epithelial cells, including rounding and detachment, especially during the interaction with L. prolificans. Plasma membrane injuries were detected in A549 cells after 1 h of co-culturing with S. aurantiacum and S. minutisporum and after 4 h with S. apiospermum and L. prolificans, as judged by the passive incorporation of propidium iodide. After 24 h of fungi-epithelial cells interaction, only mycelia were observed covering the A549 monolayer. Interestingly, the mycelial trap induced severe damage in the A549 cells, culminating in epithelial cell death. Our results demonstrate some relevant events that occur during the contact between lung epithelial cells and Scedosporium/Lomentospora species, including conidial adhesion and hyphal growth with consequent irreversible injury on A549 cells, adding light to the infection process caused by these opportunistic and multidrug-resistant fungi.
Keywords: Adhesion; Cellular interaction; Injury; Lomentospora; Lung epithelial cells; Scedosporium.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Ramirez-Garcia A, Pellon A, Rementeria A, Buldain I, Barreto-Bergter E, Rollin-Pinheiro R, de Meirelles JV, Xisto MIDS, Ranque S, Havlicek V, Vandeputte P, Govic YL, Bouchara JP, Giraud S, Chen S, Rainer J, Alastruey-Izquierdo A, Martin-Gomez MT, López-Soria LM, Peman J, Schwarz C, Bernhardt A, Tintelnot K, Capilla J, Martin-Vicente A, Cano-Lira J, Nagl M, Lackner M, Irinyi L, Meyer W, de Hoog S, Hernando FL. Scedosporium and Lomentospora: an updated overview of underrated opportunists. Med Mycol. 2017;56:S101–D125. doi: 10.1093/mmy/myx113. - DOI - PubMed
-
- Lackner M, Guarro J. Pathogenesis of Scedosporium. Curr Fungal Infect Rep. 2013;7:326–333. doi: 10.1007/s12281-013-0157-7. - DOI
-
- Cortez KJ, Roildes E, Quiroz-Telles F, Meletiadis J, Antachopoulos C, Knudsen T, Buchanan W, Milanovich J, Sutton DA, Fothergill A, Rinaldi MG, Shea YR, Zaoutis T, Kottilil S, Walsh TJ. Infections caused by Scedosporium spp. Clin Microbiol Rev. 2008;21:157–197. doi: 10.1128/CMR.00039-07. - DOI - PMC - PubMed
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

