Water-fat Dixon cardiac magnetic resonance fingerprinting
- PMID: 31736146
- PMCID: PMC7064906
- DOI: 10.1002/mrm.28070
Water-fat Dixon cardiac magnetic resonance fingerprinting
Abstract
Purpose: Cardiac magnetic resonance fingerprinting (cMRF) has been recently introduced to simultaneously provide T1 , T2 , and M0 maps. Here, we develop a 3-point Dixon-cMRF approach to enable simultaneous water specific T1 , T2 , and M0 mapping of the heart and fat fraction (FF) estimation in a single breath-hold scan.
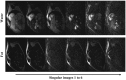
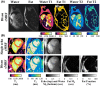
Methods: Dixon-cMRF is achieved by combining cMRF with several innovations that were previously introduced for other applications, including a 3-echo GRE acquisition with golden angle radial readout and a high-dimensional low-rank tensor constrained reconstruction to recover the highly undersampled time series images for each echo. Water-fat separation of the Dixon-cMRF time series is performed to allow for water- and fat-specific T1 , T2 , and M0 estimation, whereas FF estimation is extracted from the M0 maps. Dixon-cMRF was evaluated in a standardized T1 -T2 phantom, in a water-fat phantom, and in healthy subjects in comparison to current clinical standards: MOLLI, SASHA, T2 -GRASE, and 6-point Dixon proton density FF (PDFF) mapping.
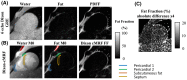
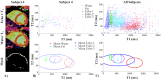
Results: Dixon-cMRF water T1 and T2 maps showed good agreement with reference T1 and T2 mapping techniques (R2 > 0.99 and maximum normalized RMSE ~5%) in a standardized phantom. Good agreement was also observed between Dixon-cMRF FF and reference PDFF (R2 > 0.99) and between Dixon-cMRF water T1 and T2 and water selective T1 and T2 maps (R2 > 0.99) in a water-fat phantom. In vivo Dixon-cMRF water T1 values were in good agreement with MOLLI and water T2 values were slightly underestimated when compared to T2 -GRASE. Average myocardium septal T1 values were 1129 ± 38 ms, 1026 ± 28 ms, and 1045 ± 32 ms for SASHA, MOLLI, and the proposed water Dixon-cMRF. Average T2 values were 51.7 ± 2.2 ms and 42.8 ± 2.6 ms for T2 -GRASE and water Dixon-cMRF, respectively. Dixon-cMRF FF maps showed good agreement with in vivo PDFF measurements (R2 > 0.98) and average FF in the septum was measured at 1.3%.
Conclusion: The proposed Dixon-cMRF allows to simultaneously quantify myocardial water T1 , water T2 , and FF in a single breath-hold scan, enabling multi-parametric T1 , T2 , and fat characterization. Moreover, reduced T1 and T2 quantification bias caused by water-fat partial volume was demonstrated in phantom experiments.
Keywords: MR fingerprinting; T1 mapping; T2 mapping; cardiac MRI; fat fraction; water-fat DIXON.
© 2019 The Authors. Magnetic Resonance in Medicine published by Wiley Periodicals, Inc. on behalf of International Society for Magnetic Resonance in Medicine.
Conflict of interest statement
Dr. Doneva, Dr. Schneider, and Mr. Koken are Philips Healthcare employees.
Figures






References
-
- Pennell DJ, Sechtem UP, Higgins CB, et al. Clinical indications for cardiovascular magnetic resonance (CMR): Consensus Panel report. Eur Heart J. 2004;25:1940–1965. - PubMed
-
- Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look‐Locker inversion recovery (MOLLI) for high‐resolution T1 mapping of the heart. Magn Reson Med. 2004;52:141–146. - PubMed
-
- Chow K, Flewitt JA, Green JD, Pagano JJ, Friedrich MG, Thompson RB. Saturation recovery single‐shot acquisition (SASHA) for myocardial T 1 mapping. Magn Reson Med. 2014;71:2082–2095. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

