Phosphoribosyl-pyrophosphate synthetase 2 (PRPS2) depletion regulates spermatogenic cell apoptosis and is correlated with hypospermatogenesis
- PMID: 31736475
- PMCID: PMC7523602
- DOI: 10.4103/aja.aja_122_19
Phosphoribosyl-pyrophosphate synthetase 2 (PRPS2) depletion regulates spermatogenic cell apoptosis and is correlated with hypospermatogenesis
Abstract
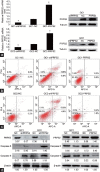
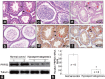
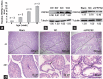
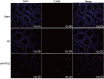
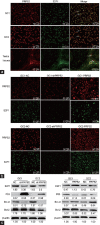
Phosphoribosyl-pyrophosphate synthetase 2 (PRPS2) is a rate-limiting enzyme and plays an important role in purine and pyrimidine nucleotide synthesis. Recent studies report that PRPS2 is involved in male infertility. However, the role of PRPS2 in hypospermatogenesis is unknown. In this study, the relationship of PRPS2 with hypospermatogenesis and spermatogenic cell apoptosis was investigated. The results showed that PRPS2 depletion increased the number of apoptotic spermatogenic cells in vitro. PRPS2 was downregulated in a mouse model of hypospermatogenesis. When PRPS2 expression was knocked down in mouse testes, hypospermatogenesis and accelerated apoptosis of spermatogenic cells were noted. E2F transcription factor 1 (E2F1) was confirmed as the target gene of PRPS2 and played a key role in cell apoptosis by regulating the P53/Bcl-xl/Bcl-2/Caspase 6/Caspase 9 apoptosis pathway. Therefore, these data indicate that PRPS2 depletion contributes to the apoptosis of spermatogenic cells and is associated with hypospermatogenesis, which may be helpful for the diagnosis of male infertility.
Keywords: hypospermatogenesis; male infertility; molecular marker; phosphoribosyl-pyrophosphate synthetase 2; spermatogenesis.
Conflict of interest statement
None
Figures

References
-
- Robin G, Boitrelle F, Leroy X, Peers MC, Marcelli F, et al. Assessment of azoospermia and histological evaluation of spermatogenesis. Ann Pathol. 2010;30:182–95. - PubMed
-
- Vogelweid CM, Verina T, Norton J, Harruff R, Ghetti B. Hypospermatogenesis is the cause of infertility in the male weaver mutant mouse. J Neurogenet. 1993;9:89–104. - PubMed
-
- Takeshima T, Yumura Y, Iwasaki A, Noguchi K. Clinical review of hypospermatogenesis in patients with a previous episode of mumps orchitis. Hinyokika Kiyo. 2015;61:227–33. Article in Japanese. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

