Insights on the relationship between structure vs. toxicological activity of antibacterial rhodamine-labelled 3-hydroxy-4-pyridinone iron(III) chelators in HepG2 cells
- PMID: 31736632
- PMCID: PMC6853001
- DOI: 10.2478/intox-2018-0016
Insights on the relationship between structure vs. toxicological activity of antibacterial rhodamine-labelled 3-hydroxy-4-pyridinone iron(III) chelators in HepG2 cells
Abstract
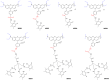
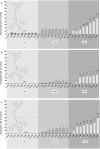
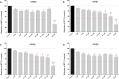
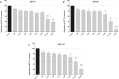
In the present study we investigated the in vitro hepatotoxicity of a set of rhodamine-labelled 3-hydroxy-4-pyridinones (3,4-HPO) that had previously demonstrated significant inhibitory effect in the intramacrophagic growth of Mycobacterium avium. Our aim was to establish a correspondence between the molecular structure and the in vitro toxicological activity of these compounds. The impact of a set of bidentate (MRB2, MRB7, MRB8, and MRB9) and hexadentate (MRH7, MRH8, and MRH10) chelators on cellular metabolic competence and membrane integrity was investigated in HepG2 cells. Our findings indicate that: a) hexadentate chelators are more cytotoxic than parent bidentate ligands; b) disruption of cell membrane and metabolic competence only occurred after 5 days, at the highest concentrations tested; c) strict correlation between bacteriostatic activity and in vitro toxicity was observed, which seems to be directly dependent on the size of the molecule and on the hydrophilic/lipophilic balance; d) among the set of bidentate ligands, carboxyrhodamine derivatives (amide linker) presented lower detrimental effects, when compared with rhodamine B isothiocyanate chelators (thiourea linker); e) contrarily, for the hexadentate series, rhodamine B isothiocyanate derivatives are less cytotoxic to HepG2 cells than carboxyrhodamine molecules; and f) for all compounds tested, when the substituents of the nitrogen atom were switched from ethyl to methyl, an increment of toxicity was observed. Overall, all chelators seem to display suitable in vitro toxicological potential to combat fast grow bacteria. According to their in vitro pharmacological: toxicological potential ratio, MRH7 and MRH8 may be considered as the most suitable compounds to undergo further pre-clinical development studies.
Keywords: 3-hydroxy-4-pyridinone (3,4-HPO); HepG2 cells; in vitrotoxicity; iron chelator; rhodamine.
Copyright © 2018 SETOX & Institute of Experimental Pharmacology and Toxicology, SASc.
Figures


References
-
- al-Khafaji B, Kralovic S, Smith RD. Increased hepatic iron in the acquired immunodeficiency syndrome: an autopsy study. Mod Pathol. 1997;10:474–480. - PubMed
-
- Amit A, Chaudhary R, Yadav A, Suman SS, Narayan S, Das VN, Pandey K, Singh SK, Singh BK, Ali V, Das P, Bimal S. Evaluation of Leishmania donovani disulfide isomerase as a potential target of cellular immunity against visceral leishmaniasis. Cell Immunol. 2014;289:76–85. - PubMed
-
- Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annu Rev. 2005;11:127–152. - PubMed
-
- Brown DM, Johnston H, Gubbins E, Stone V. Serum enhanced cytokine responses of macrophages to silica and iron oxide particles and nanomaterials: a comparison of serum to lung lining fluid and albumin dispersions. J Appl Toxicol. 2014;34:1177–1187. - PubMed
-
- Coimbra JT, Moniz T, Brás NF, Ivanova G, Fernandes PA, Ramos MJ, Rangel M. Relevant interactions of antimicrobial iron chelators and membrane models revealed by nuclear magnetic resonance and molecular dynamics simulations. J Phys Chem B. 2014;118:14590–14601. - PubMed
LinkOut - more resources
Full Text Sources
