Dye Tracking Following Posterior Semicircular Canal or Round Window Membrane Injections Suggests a Role for the Cochlea Aqueduct in Modulating Distribution
- PMID: 31736710
- PMCID: PMC6833940
- DOI: 10.3389/fncel.2019.00471
Dye Tracking Following Posterior Semicircular Canal or Round Window Membrane Injections Suggests a Role for the Cochlea Aqueduct in Modulating Distribution
Abstract
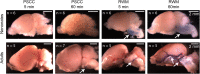
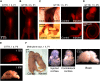
The inner ear houses the sensory epithelium responsible for vestibular and auditory function. The sensory epithelia are driven by pressure and vibration of the fluid filled structures in which they are embedded so that understanding the homeostatic mechanisms regulating fluid dynamics within these structures is critical to understanding function at the systems level. Additionally, there is a growing need for drug delivery to the inner ear for preventive and restorative treatments to the pathologies associated with hearing and balance dysfunction. We compare drug delivery to neonatal and adult inner ear by injection into the posterior semicircular canal (PSCC) or through the round window membrane (RWM). PSCC injections produced higher levels of dye delivery within the cochlea than did RWM injections. Neonatal PSCC injections produced a gradient in dye distribution; however, adult distributions were relatively uniform. RWM injections resulted in an early base to apex gradient that became more uniform over time, post injection. RWM injections lead to higher levels of dye distributions in the brain, likely demonstrating that injections can traverse the cochlea aqueduct. We hypothesize the relative position of the cochlear aqueduct between injection site and cochlea is instrumental in dictating dye distribution within the cochlea. Dye distribution is further compounded by the ability of some chemicals to cross inner ear membranes accessing the blood supply as demonstrated by the rapid distribution of gentamicin-conjugated Texas red (GTTR) throughout the body. These data allow for a direct evaluation of injection mode and age to compare strengths and weaknesses of the two approaches.
Keywords: cochlea aqueduct; drug delivery; endolymph; inner ear; perilymph; posterior semicircular canal; round window.
Copyright © 2019 Talaei, Schnee, Aaron and Ricci.
Figures






Similar articles
-
Round window membrane intracochlear drug delivery enhanced by induced advection.J Control Release. 2014 Jan 28;174:171-6. doi: 10.1016/j.jconrel.2013.11.021. Epub 2013 Dec 1. J Control Release. 2014. PMID: 24291333 Free PMC article.
-
Semicircular canal drug delivery safely targets the inner ear perilymphatic space.JCI Insight. 2024 Nov 8;9(21):e173052. doi: 10.1172/jci.insight.173052. JCI Insight. 2024. PMID: 39513368 Free PMC article.
-
Membrane curvature and connective fiber alignment in guinea pig round window membrane.Acta Biomater. 2021 Dec;136:343-362. doi: 10.1016/j.actbio.2021.09.036. Epub 2021 Sep 24. Acta Biomater. 2021. PMID: 34563725 Free PMC article.
-
Review of round window membrane permeability.Acta Otolaryngol Suppl. 1989;457:43-8. doi: 10.3109/00016488809138883. Acta Otolaryngol Suppl. 1989. PMID: 2648755 Review.
-
Cochlear implantation in children with anomalous cochleovestibular anatomy.Laryngoscope. 2005 Jan;115(1 Pt 2 Suppl 106):1-26. doi: 10.1097/00005537-200501001-00001. Laryngoscope. 2005. PMID: 15626926 Review.
Cited by
-
Gene Therapy for Inherited Hearing Loss: Updates and Remaining Challenges.Audiol Res. 2023 Dec 4;13(6):952-966. doi: 10.3390/audiolres13060083. Audiol Res. 2023. PMID: 38131808 Free PMC article. Review.
-
Outer hair cells stir cochlear fluids.bioRxiv [Preprint]. 2024 Nov 12:2024.08.07.607009. doi: 10.1101/2024.08.07.607009. bioRxiv. 2024. Update in: Elife. 2025 Jan 16;13:RP101943. doi: 10.7554/eLife.101943. PMID: 39149246 Free PMC article. Updated. Preprint.
-
Improved optogenetic modification of spiral ganglion neurons for future optical cochlear implants.Theranostics. 2025 Mar 18;15(10):4270-4286. doi: 10.7150/thno.104474. eCollection 2025. Theranostics. 2025. PMID: 40225583 Free PMC article.
-
Virally mediated enhancement of efferent inhibition reduces acoustic trauma in wild-type murine cochleas.Mol Ther Methods Clin Dev. 2025 Mar 21;33(2):101455. doi: 10.1016/j.omtm.2025.101455. eCollection 2025 Jun 12. Mol Ther Methods Clin Dev. 2025. PMID: 40236498 Free PMC article.
-
In utero adeno-associated virus (AAV)-mediated gene delivery targeting sensory and supporting cells in the embryonic mouse inner ear.PLoS One. 2024 Jul 19;19(7):e0305742. doi: 10.1371/journal.pone.0305742. eCollection 2024. PLoS One. 2024. PMID: 39028743 Free PMC article.
References
-
- Carlborg B. (1981). On physiological and experimental variation of the perilymphatic pressure in the cat. Acta Otolaryngol. 91 19–28. 10.3109/00016488109138478 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

