Induction of Tolerance and Immunity by Dendritic Cells: Mechanisms and Clinical Applications
- PMID: 31736936
- PMCID: PMC6830192
- DOI: 10.3389/fimmu.2019.02393
Induction of Tolerance and Immunity by Dendritic Cells: Mechanisms and Clinical Applications
Abstract
Dendritic cells (DCs) are key regulators of immune responses that operate at the interface between innate and adaptive immunity, and defects in DC functions contribute to the pathogenesis of a variety of disorders. For instance, cancer evolves in the context of limited DC activity, and some autoimmune diseases are initiated by DC-dependent antigen presentation. Thus, correcting aberrant DC functions stands out as a promising therapeutic paradigm for a variety of diseases, as demonstrated by an abundant preclinical and clinical literature accumulating over the past two decades. However, the therapeutic potential of DC-targeting approaches remains to be fully exploited in the clinic. Here, we discuss the unique features of DCs that underlie the high therapeutic potential of DC-targeting strategies and critically analyze the obstacles that have prevented the full realization of this promising paradigm.
Keywords: autoimmune disorders; cancer; dendritic cells; immunotherapy; vaccine preparation.
Copyright © 2019 Fucikova, Palova-Jelinkova, Bartunkova and Spisek.
Figures

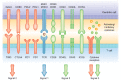
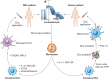
References
-
- Schreibelt G, Tel J, Sliepen KH, Benitez-Ribas D, Figdor CG, Adema GJ, et al. . Toll-like receptor expression and function in human dendritic cell subsets: implications for dendritic cell-based anti-cancer immunotherapy. Cancer Immunol Immunother. (2010) 59:1573–82. 10.1007/s00262-010-0833-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

