Evaluation of the Effect Derived from Silybin with Vitamin D and Vitamin E Administration on Clinical, Metabolic, Endothelial Dysfunction, Oxidative Stress Parameters, and Serological Worsening Markers in Nonalcoholic Fatty Liver Disease Patients
- PMID: 31737175
- PMCID: PMC6815609
- DOI: 10.1155/2019/8742075
Evaluation of the Effect Derived from Silybin with Vitamin D and Vitamin E Administration on Clinical, Metabolic, Endothelial Dysfunction, Oxidative Stress Parameters, and Serological Worsening Markers in Nonalcoholic Fatty Liver Disease Patients
Abstract
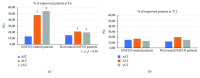
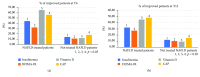
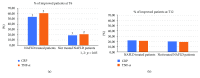
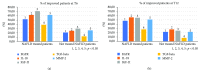
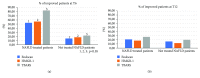
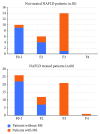
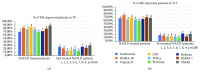
Nowadays, the nonalcoholic fatty liver disease represents the main chronic liver disease in the Western countries, and the correct medical therapy remains a big question for the scientific community. The aim of our study was to evaluate the effect derived from the administration for six months of silybin with vitamin D and vitamin E (RealSIL 100D®) on metabolic markers, oxidative stress, endothelial dysfunction, and worsening of disease markers in nonalcoholic fatty liver disease patients. We enrolled 90 consecutive patients with histological diagnosis of nonalcoholic fatty liver disease and 60 patients with diagnosis of reflux disease (not in therapy) as healthy controls. The nonalcoholic fatty liver disease patients were randomized into two groups: treated (60 patients) and not treated (30 patients). We performed a nutritional assessment and evaluated clinical parameters, routine home tests, the homeostatic model assessment of insulin resistance, NAFLD fibrosis score and fibrosis-4, transient elastography and controlled attenuation parameter, thiobarbituric acid reactive substances, tumor necrosis factor α, transforming growth factor β, interleukin-18 and interleukin-22, matrix metalloproteinase 2, epidermal growth factor receptor, insulin growth factor-II, cluster of differentiation-44, high mobility group box-1, and Endocan. Compared to the healthy controls, the nonalcoholic fatty liver disease patients had statistically significant differences for almost all parameters evaluated at baseline (p < 0.05). Six months after the baseline, the proportion of nonalcoholic fatty liver disease patients treated that underwent a statistically significant improvement in metabolic markers, oxidative stress, endothelial dysfunction, and worsening of disease was greater than not treated nonalcoholic fatty liver disease patients (p < 0.05). Even more relevant results were obtained for the same parameters by analyzing patients with a concomitant diagnosis of metabolic syndrome (p < 0.001). The benefit that derives from the use of RealSIL 100D could derive from the action on more systems able to advance the pathology above all in that subset of patients suffering from concomitant metabolic syndrome.
Copyright © 2019 Alessandro Federico et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Federico A., Dallio M., Masarone M., Persico M., Loguercio C. The epidemiology of non-alcoholic fatty liver disease and its connection with cardiovascular disease: role of endothelial dysfunction. European Review for Medical and Pharmacological Sciences. 2016;20(22):4731–4741. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

