Survival score to characterize prognosis in inoperable stage III NSCLC after chemoradiotherapy
- PMID: 31737496
- PMCID: PMC6835102
- DOI: 10.21037/tlcr.2019.09.19
Survival score to characterize prognosis in inoperable stage III NSCLC after chemoradiotherapy
Abstract
Background: Stage III non-small cell lung cancer (NSCLC) represents a heterogeneous disease regarding principal patient- and tumor characteristics. A simple score may aid in personalizing multimodal therapy.
Methods: The data of 99 consecutive patients with performance status ECOG 0-1 treated until the end of 2016 with multimodal approach for inoperable NSCLC (UICC 7th edition stage IIIA/B) were evaluated. Patient- and tumor-related factors were examined for their impact on overall survival. Factors showing a negative association with prognosis were then included in the score. Three subgroups with low, intermediate and high-risk score were defined. The results were then validated in the prospective cohort, which includes 45 patients.
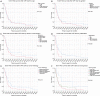
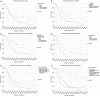
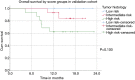
Results: Most Patients were treated with concurrent (78%) or sequential (11%) chemoradiotherapy. 53% received induction chemotherapy. Median survival for the entire cohort was 20.8 (range: 15.3-26.3) months. Age (P=0.020), gender (P=0.007), pack years (P=0.015), tumor-associated atelectasis (P=0.004) and histology (P=0.004) had a significant impact on overall survival and were scored with one point each. Twelve, 59 and 28 patients were defined to have a low (0-1 points), intermediate (2-3 points) and high-risk (4-5 points) score. Median survival, 1-, 2- and 3-year survival rates were not reached, 100%, 83% and 67% in the low, 22.9 months, 80%, 47% and 24% intermediate and 13.7 months, 57%, 25% and 18% high-risk patients, respectively (P<0.001). Median survival was not reached in prospective cohort; analysis has revealed a trend for the 1-year survival rates with 100% for the low, 93% intermediate and 69% high-risk patients (P=0.100).
Conclusions: The score demonstrated remarkable survival differences in inoperable stage III NSCLC patients with good performance status receiving multimodal therapy.
Keywords: Non-small cell lung cancer (NSCLC); multimodal therapy; prognostic factors; survival-score.
2019 Translational Lung Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures
References
-
- W. SB, P. WC. World Cancer Report 2014. Geneva: World Health Organization; 2014. Available online: http://gbv.eblib.com/patron/FullRecord.aspx?p=1635715
-
- Robert Koch-Institut. Krebs in Deutschland für 2013/2014.
