Dihydroergotamine (DHE) - Then and Now: A Narrative Review
- PMID: 31737909
- PMCID: PMC7003832
- DOI: 10.1111/head.13700
Dihydroergotamine (DHE) - Then and Now: A Narrative Review
Abstract
Objective: To provide a narrative review of clinical development programs for non-oral, non-injectable formulations of dihydroergotamine (DHE) for the treatment of migraine.
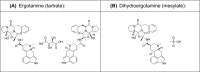
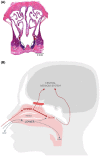
Background: Dihydroergotamine was one of the first "synthetic drugs" developed in the 20th century for treating migraine. It is effective and recommended for acute migraine treatment. Since oral DHE is extensively metabolized, it must be given by a non-oral route. Intravenous DHE requires healthcare personnel to administer, subcutaneous/intramuscular injection is challenging to self-administer, and the approved nasal spray formulation exhibits low bioavailability and high variability that limits its efficacy. Currently there are several attempts underway to develop non-oral, non-injected formulations of DHE.
Method: A systematic search of MEDLINE/PubMed and ClinicalTrials.gov databases, then narrative review of identified reports, focusing on those published in the last 10 years.
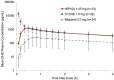
Results: Of 1881 references to DHE from a MEDLINE/PubMed search, 164 were from the last 10 years and were the focus of this review. Further cross reference was made to ClinicalTrials.gov for 19 clinical studies, of which some results have not yet been published, or are studies that are currently underway. Three nasal DHE products are in clinical development, reawakening interest in this route of delivery for migraine. Other routes of DHE administration have been, or are being, explored.
Conclusion: There is renewed appreciation for DHE and the need for non-oral, non-injected delivery is now being addressed.
Keywords: acute migraine; clinical; dihydroergotamine; intranasal delivery.
© 2019 The Authors. Headache: The Journal of Head and Face Pain published by Wiley Periodicals, Inc. on behalf of American Headache Society.
Figures


References
-
- Tfelt‐Hansen P, Saxena PR, Dahlöf C, et al. Ergotamine in the acute treatment of migraine: A review and European consensus. Brain. 2000;123(Pt 1):9‐18. - PubMed
-
- Lipton RB, Ergotamine tartrate and dihydroergotamine mesylate: Safety profiles. Headache. 1997;37:S33‐S41. - PubMed
-
- Horton BT, Peters GA, Blumenthal LS. A new product in the treatment of migraine: A preliminary report. Mayo Clin Proc. 1945;20:241‐248.
-
- Silberstein SD, McCrory DC. Ergotamine and dihydroergotamine: History, pharmacology, and efficacy. Headache. 2003;43:144‐166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

