Post-ejaculatory modifications to sperm (PEMS)
- PMID: 31737992
- PMCID: PMC7643048
- DOI: 10.1111/brv.12569
Post-ejaculatory modifications to sperm (PEMS)
Abstract
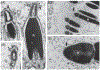
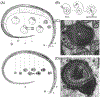
Mammalian sperm must spend a minimum period of time within a female reproductive tract to achieve the capacity to fertilize oocytes. This phenomenon, termed sperm 'capacitation', was discovered nearly seven decades ago and opened a window into the complexities of sperm-female interaction. Capacitation is most commonly used to refer to a specific combination of processes that are believed to be widespread in mammals and includes modifications to the sperm plasma membrane, elevation of intracellular cyclic AMP levels, induction of protein tyrosine phosphorylation, increased intracellular Ca2+ levels, hyperactivation of motility, and, eventually, the acrosome reaction. Capacitation is only one example of post-ejaculatory modifications to sperm (PEMS) that are widespread throughout the animal kingdom. Although PEMS are less well studied in non-mammalian taxa, they likely represent the rule rather than the exception in species with internal fertilization. These PEMS are diverse in form and collectively represent the outcome of selection fashioning complex maturational trajectories of sperm that include multiple, sequential phenotypes that are specialized for stage-specific functionality within the female. In many cases, PEMS are critical for sperm to migrate successfully through the female reproductive tract, survive a protracted period of storage, reach the site of fertilization and/or achieve the capacity to fertilize eggs. We predict that PEMS will exhibit widespread phenotypic plasticity mediated by sperm-female interactions. The successful execution of PEMS thus has important implications for variation in fitness and the operation of post-copulatory sexual selection. Furthermore, it may provide a widespread mechanism of reproductive isolation and the maintenance of species boundaries. Despite their possible ubiquity and importance, the investigation of PEMS has been largely descriptive, lacking any phylogenetic consideration with regard to divergence, and there have been no theoretical or empirical investigations of their evolutionary significance. Here, we (i) clarify PEMS-related nomenclature; (ii) address the evolutionary origin, maintenance and divergence in PEMS in the context of the protracted life history of sperm and the complex, selective environment of the female reproductive tract; (iii) describe taxonomically widespread types of PEMS: sperm activation, chemotaxis and the dissociation of sperm conjugates; (iv) review the occurence of PEMS throughout the animal kingdom; (v) consider alternative hypotheses for the adaptive value of PEMS; (vi) speculate on the evolutionary implications of PEMS for genomic architecture, sexual selection, and reproductive isolation; and (vii) suggest fruitful directions for future functional and evolutionary analyses of PEMS.
Keywords: capacitation; female reproductive tract; fertility; hyperactivation; morphogenesis; motility; post-copulatory sexual selection; seminal proteins; sperm competition; spermatozoa.
© 2019 Cambridge Philosophical Society.
Figures




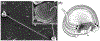

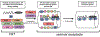


References
-
- Fuentes-G JA, Housworth EA, Weber A & Martins EP (2016). Phylogenetic ANCOVA: estimating changes in evolutionary rates as well as relationships between traits. The American Naturalist 188, 615–627. - PubMed
-
- Aalberts M, Stout TAE & Stoorvogel W, (2014). Prostasomes: extracellular vesicles from the prostate. Reproduction 147, R1–R14. - PubMed
-
- Adams DC (2014). Quantifying and comparing phylogenetic evolutionary rates for shape and other high-dimensional phenotypic data. Systematic Biology 63, 166–177. - PubMed
-
- Afzelius BA & Dallai R (1987). Conjugated spermatozoa In New Horizons in Sperm Cell Research (ed. Mohri H), pp. 349–355. Japan Science Society, Tokyo.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

