Increased Moraxella and Streptococcus species abundance after severe bronchiolitis is associated with recurrent wheezing
- PMID: 31738994
- PMCID: PMC7010548
- DOI: 10.1016/j.jaci.2019.10.034
Increased Moraxella and Streptococcus species abundance after severe bronchiolitis is associated with recurrent wheezing
Abstract
Background: The role of the airway microbiome in the development of recurrent wheezing and asthma remains uncertain, particularly in the high-risk group of infants hospitalized for bronchiolitis.
Objective: We sought to examine the relation of the nasal microbiota at bronchiolitis-related hospitalization and 3 later points to the risk of recurrent wheezing by age 3 years.
Methods: In 17 US centers researchers collected clinical data and nasal swabs from infants hospitalized for bronchiolitis. Trained parents collected nasal swabs 3 weeks after hospitalization and, when healthy, during the summer and 1 year after hospitalization. We applied 16S rRNA gene sequencing to all nasal swabs. We used joint modeling to examine the relation of longitudinal nasal microbiota abundances to the risk of recurrent wheezing.
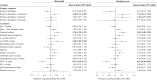
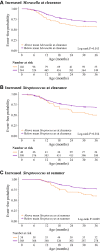
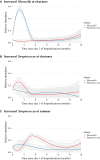
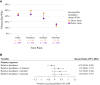
Results: Among 842 infants hospitalized for bronchiolitis, there was 88% follow-up at 3 years, and 31% had recurrent wheezing. The median age at enrollment was 3.2 months (interquartile range, 1.7-5.8 months). In joint modeling analyses adjusting for 16 covariates, including viral cause, a 10% increase in relative abundance of Moraxella or Streptococcus species 3 weeks after day 1 of hospitalization was associated with an increased risk of recurrent wheezing (hazard ratio [HR] of 1.38 and 95% high-density interval [HDI] of 1.11-1.85 and HR of 1.76 and 95% HDI of 1.13-3.19, respectively). Increased Streptococcus species abundance the summer after hospitalization was also associated with a greater risk of recurrent wheezing (HR, 1.76; 95% HDI, 1.15-3.27).
Conclusions: Enrichment of Moraxella or Streptococcus species after bronchiolitis hospitalization was associated with recurrent wheezing by age 3 years, possibly providing new avenues to ameliorate the long-term respiratory outcomes of infants with severe bronchiolitis.
Keywords: Bronchiolitis; Haemophilus species; Moraxella species; Streptococcus species; longitudinal studies; microbiome; recurrent wheezing; respiratory syncytial virus; rhinovirus.
Copyright © 2019 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Hasegawa K., Mansbach J.M., Camargo C.A., Jr. Infectious pathogens and bronchiolitis outcomes. Expert Rev Anti Infect Ther. 2014;12:817–828. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

