The Role of Vascular Smooth Muscle Cells in Arterial Remodeling: Focus on Calcification-Related Processes
- PMID: 31739395
- PMCID: PMC6888164
- DOI: 10.3390/ijms20225694
The Role of Vascular Smooth Muscle Cells in Arterial Remodeling: Focus on Calcification-Related Processes
Abstract
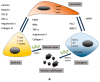
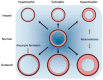
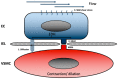
Arterial remodeling refers to the structural and functional changes of the vessel wall that occur in response to disease, injury, or aging. Vascular smooth muscle cells (VSMC) play a pivotal role in regulating the remodeling processes of the vessel wall. Phenotypic switching of VSMC involves oxidative stress-induced extracellular vesicle release, driving calcification processes. The VSMC phenotype is relevant to plaque initiation, development and stability, whereas, in the media, the VSMC phenotype is important in maintaining tissue elasticity, wall stress homeostasis and vessel stiffness. Clinically, assessment of arterial remodeling is a challenge; particularly distinguishing intimal and medial involvement, and their contributions to vessel wall remodeling. The limitations pertain to imaging resolution and sensitivity, so methodological development is focused on improving those. Moreover, the integration of data across the microscopic (i.e., cell-tissue) and macroscopic (i.e., vessel-system) scale for correct interpretation is innately challenging, because of the multiple biophysical and biochemical factors involved. In the present review, we describe the arterial remodeling processes that govern arterial stiffening, atherosclerosis and calcification, with a particular focus on VSMC phenotypic switching. Additionally, we review clinically applicable methodologies to assess arterial remodeling and the latest developments in these, seeking to unravel the ubiquitous corroborator of vascular pathology that calcification appears to be.
Keywords: arterial remodeling; hypertension; phenotype switching; vascular calcification; vascular smooth muscle cell; vascular stiffness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Schurgers L.J., Akbulut A.C., Kaczor D.M., Halder M., Koenen R.R., Kramann R. Initiation and Propagation of Vascular Calcification Is Regulated by a Concert of Platelet- and Smooth Muscle Cell-Derived Extracellular Vesicles. Front. Cardiovasc. Med. 2018;5:36. doi: 10.3389/fcvm.2018.00036. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

