Prebiotic Chemistry that Could Not Not Have Happened
- PMID: 31739415
- PMCID: PMC6958414
- DOI: 10.3390/life9040084
Prebiotic Chemistry that Could Not Not Have Happened
Abstract
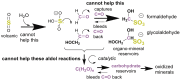
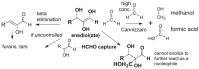
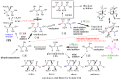
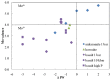
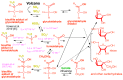
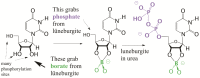
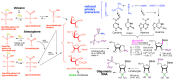
We present a direct route by which RNA might have emerged in the Hadean from a fayalite-magnetite mantle, volcanic SO2 gas, and well-accepted processes that must have created substantial amounts of HCHO and catalytic amounts of glycolaldehyde in the Hadean atmosphere. In chemistry that could not not have happened, these would have generated stable bisulfite addition products that must have rained to the surface, where they unavoidably would have slowly released reactive species that generated higher carbohydrates. The formation of higher carbohydrates is self-limited by bisulfite formation, while borate minerals may have controlled aldol reactions that occurred on any semi-arid surface to capture that precipitation. All of these processes have well-studied laboratory correlates. Further, any semi-arid land with phosphate should have had phosphate anhydrides that, with NH3, gave carbohydrate derivatives that directly react with nucleobases to form the canonical nucleosides. These are phosphorylated by magnesium borophosphate minerals (e.g., lüneburgite) and/or trimetaphosphate-borate with Ni2+ catalysis to give nucleoside 5'-diphosphates, which oligomerize to RNA via a variety of mechanisms. The reduced precursors that are required to form the nucleobases came, in this path-hypothesis, from one or more mid-sized (1023-1020 kg) impactors that almost certainly arrived after the Moon-forming event. Their iron metal content almost certainly generated ammonia, nucleobase precursors, and other reduced species in the Hadean atmosphere after it transiently placed the atmosphere out of redox equilibrium with the mantle. In addition to the inevitability of steps in this path-hypothesis on a Hadean Earth if it had semi-arid land, these processes may also have occurred on Mars. Adapted from a lecture by the Corresponding Author at the All-Russia Science Festival at the Lomonosov Moscow State University on 12 October 2019, and is an outcome of a three year project supported by the John Templeton Foundation and the NASA Astrobiology program. Dedicated to David Deamer, on the occasion of his 80th Birthday.
Keywords: Hadean; RNA formation; meteorite impact; organic minerals; prebiotic chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Gilbert W. Origin of life: The RNA world. Nature. 1986;319:618. doi: 10.1038/319618a0. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

