5,8-Quinolinedione Scaffold as a Promising Moiety of Bioactive Agents
- PMID: 31739496
- PMCID: PMC6891355
- DOI: 10.3390/molecules24224115
5,8-Quinolinedione Scaffold as a Promising Moiety of Bioactive Agents
Abstract
Natural 5,8-quinolinedione antibiotics exhibit a broad spectrum of activities including anticancer, antibacterial, antifungal, and antimalarial activities. The structure-activity research showed that the 5,8-quinolinedione scaffold is responsible for its biological effect. The subject of this review report is a presentation of the pharmacological activity of synthetic 5,8-quinolinedione compounds containing different groups at C-6 and/or C-7 positions. The relationship between the activity and the mechanism of action is included if these data have been included in the original literature. The review mostly covers the period between 2000 and 2019. Previously published literature data were used to present historical points.
Keywords: 5,8-quinolinedione; NAD[P]H-quinone oxidoreductase; biological activity; streptonigrin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








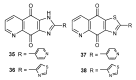

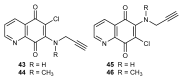
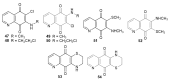
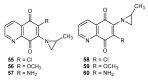
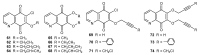

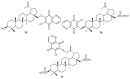

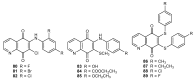
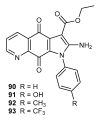





References
-
- Rao K.V., Cullen W.P. Streptonigrin, an antitumor substance. I. Isolation and characterization. Antibiot. Annu. 1959;7:950–953. - PubMed
-
- Kudrina E.S., Ol’khovatova O.L., Murav’eva L.I., Gauze G.F. Systematic position and variability of the producent of antitumor antibiotic bruneomycin. Antibiotiki (Mocsow) 1966;11:400–405. - PubMed
-
- Societe des Usines Chemiques Rhone-Poulenc. 8,722,61. British Patent. 1961 Jul 5;
-
- Rao K.V., Biemann K., Woodward R.B. The structure of Streptonigrin. J. Am. Chem. Soc. 1963;85:2532–2533. doi: 10.1021/ja00899a051. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

