Neuronal maturation reduces the type I IFN response to orthobunyavirus infection and leads to increased apoptosis of human neurons
- PMID: 31739796
- PMCID: PMC6862864
- DOI: 10.1186/s12974-019-1614-1
Neuronal maturation reduces the type I IFN response to orthobunyavirus infection and leads to increased apoptosis of human neurons
Abstract
Background: La Crosse virus (LACV) is the leading cause of pediatric arboviral encephalitis in the USA. LACV encephalitis can result in learning and memory deficits, which may be due to infection and apoptosis of neurons in the brain. Despite neurons being the primary cell infected in the brain by LACV, little is known about neuronal responses to infection.
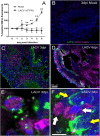
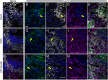
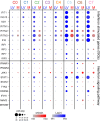
Methods: Human cerebral organoids (COs), which contain a spectrum of developing neurons, were used to examine neuronal responses to LACV. Plaque assay and quantitative reverse transcription (qRT) PCR were used to determine the susceptibility of COs to LACV infection. Immunohistochemistry, flow cytometry, and single-cell transcriptomics were used to determine specific neuronal subpopulation responses to the virus.
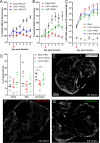
Results: Overall, LACV readily infected COs causing reduced cell viability and increased apoptosis. However, it was determined that neurons at different stages of development had distinct responses to LACV. Both neural progenitors and committed neurons were infected with LACV, however, committed neurons underwent apoptosis at a higher rate. Transcriptomic analysis showed that committed neurons expressed fewer interferon (IFN)-stimulated genes (ISGs) and genes involved IFN signaling in response to infection compared to neural progenitors. Furthermore, induction of interferon signaling in LACV-infected COs by application of recombinant IFN enhanced cell viability.
Conclusions: These findings indicate that neuronal maturation increases the susceptibility of neurons to LACV-induced apoptosis. This susceptibility is likely due, at least in part, to mature neurons being less responsive to virus-induced IFN as evidenced by their poor ISG response to LACV. Furthermore, exogenous administration of recombinant IFN to LACV COs rescued cellular viability suggesting that increased IFN signaling is overall protective in this complex neural tissue. Together these findings indicate that induction of IFN signaling in developing neurons is an important deciding factor in virus-induced cell death.
Keywords: Apoptosis; Encephalitis; Human cerebral organoids; Neurogenesis; Neuron; Orthobunyavirus; Single-cell transcriptomics; Type I interferon.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- La Crosse Encephalitis Epidemiology & Geographic Distribution. [https://www.cdc.gov/lac/tech/epi.html]. Accessed 1 July 2019.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

