Estimating the impact of multiple immunization products on medically-attended respiratory syncytial virus (RSV) infections in infants
- PMID: 31740097
- PMCID: PMC7029767
- DOI: 10.1016/j.vaccine.2019.10.023
Estimating the impact of multiple immunization products on medically-attended respiratory syncytial virus (RSV) infections in infants
Abstract
Background: Palivizumab, a monoclonal antibody and the only licensed immunization product for preventing respiratory syncytial virus (RSV) infection, is recommended for children with certain high-risk conditions. Other antibody products and maternal vaccines targeting young infants are in clinical development. Few studies have compared products closest to potential licensure and have primarily focused on the effects on hospitalizations only. Estimates of the impact of these products on medically-attended (MA) infections in a variety of healthcare settings are needed to assist with developing RSV immunization recommendations.
Methods: We developed a tool for practicing public health officials to estimate the impact of immunization strategies on RSV-associated MA lower respiratory tract infections (LRTIs) in various healthcare settings among infants <12 months. Users input RSV burden and seasonality and examine the influence of altering product efficacy and uptake assumptions. We used the tool to evaluate candidate products' impacts among a US birth cohort.
Results: We estimated without immunization, 407,360 (range: 339,650-475,980) LRTIs are attended annually in outpatient clinics, 147,240 (126,070-168,510) in emergency departments (EDs), and 33,180 (24,760-42,900) in hospitals. A passive antibody candidate targeting all infants prevented the most LRTIs: 196,470 (48% of visits without immunization) outpatient clinic visits (range: 163,810-229,650), 75,250 (51%) EDs visits (64,430-86,090), and 18,140 (55%) hospitalizations (13,770-23,160). A strategy combining maternal vaccine candidate and palivizumab prevented 58,210 (14% of visits without immunization) LRTIs in outpatient clinics (range: 48,520-67,970), 19,580 (13%) in EDs (16,760-22,400), and 8,190 (25%) hospitalizations (6,390-10,150).
Conclusions: Results underscore the potential for anticipated products to reduce serious RSV illness. Our tool (provided to readers) can be used by different jurisdictions and accept updated data. Results can aid economic evaluations and public health decision-making regarding RSV immunization products.
Keywords: Infants; Maternal vaccination; Model; Passive immunization; RSV; United States.
Published by Elsevier Ltd.
Conflict of interest statement
Declaration of interests:
The findings and conclusions in the report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
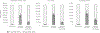
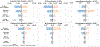
References
-
- Lively JY et al. Respiratory syncytial virus-associated outpatient visits among children younger than 24 months. J Pediatric Infect Dis Soc 2019. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

