NurOwn, phase 2, randomized, clinical trial in patients with ALS: Safety, clinical, and biomarker results
- PMID: 31740545
- PMCID: PMC6937497
- DOI: 10.1212/WNL.0000000000008620
NurOwn, phase 2, randomized, clinical trial in patients with ALS: Safety, clinical, and biomarker results
Abstract
Objective: To determine the safety and efficacy of mesenchymal stem cell (MSC)-neurotrophic factor (NTF) cells (NurOwn®, autologous bone marrow-derived MSCs, induced to secrete NTFs) delivered by combined intrathecal and intramuscular administration to participants with amyotrophic lateral sclerosis (ALS) in a phase 2 randomized controlled trial.
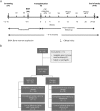
Methods: The study enrolled 48 participants randomized 3:1 (treatment: placebo). After a 3-month pretransplant period, participants received 1 dose of MSC-NTF cells (n = 36) or placebo (n = 12) and were followed for 6 months. CSF was collected before and 2 weeks after transplantation.
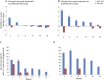
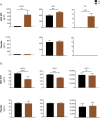
Results: The study met its primary safety endpoint. The rate of disease progression (Revised ALS Functional Rating Scale [ALSFRS-R] slope change) in the overall study population was similar in treated and placebo participants. In a prespecified rapid progressor subgroup (n = 21), rate of disease progression was improved at early time points (p < 0.05). To address heterogeneity, a responder analysis showed that a higher proportion of treated participants experienced ≥1.5 points/month ALSFRS-R slope improvement compared to placebo at all time points, and was significant in rapid progressors at 4 and 12 weeks (p = 0.004 and 0.046, respectively). CSF neurotrophic factors increased and CSF inflammatory biomarkers decreased in treated participants (p < 0.05) post-transplantation. CSF monocyte chemoattractant protein-1 levels correlated with ALSFRS-R slope improvement up to 24 weeks (p < 0.05).
Conclusion: A single-dose transplantation of MSC-NTF cells is safe and demonstrated early promising signs of efficacy. This establishes a clear path forward for a multidose randomized clinical trial of intrathecal autologous MSC-NTF cell transplantation in ALS.
Classification of evidence: This phase II study provides Class I evidence.
Copyright © 2019 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures

Comment in
-
Stem cells in amyotrophic lateral sclerosis: Hype or hope?Neurology. 2019 Dec 10;93(24):1028-1029. doi: 10.1212/WNL.0000000000008613. Epub 2019 Nov 18. Neurology. 2019. PMID: 31740544 No abstract available.
References
-
- Brown RH, Al-Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med 2017;377:162–172. - PubMed
-
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008;8:726–736. - PubMed
-
- Petrou P, Gothelf Y, Argov Z, et al. Safety and clinical effects of mesenchymal stem cells secreting neurotrophic factor transplantation in patients with amyotrophic lateral sclerosis: results of phase 1/2 and 2a clinical trials. JAMA Neurol 2016;73:337–344. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
