Biofilms of Mycobacterium abscessus Complex Can Be Sensitized to Antibiotics by Disaggregation and Oxygenation
- PMID: 31740557
- PMCID: PMC6985723
- DOI: 10.1128/AAC.01212-19
Biofilms of Mycobacterium abscessus Complex Can Be Sensitized to Antibiotics by Disaggregation and Oxygenation
Abstract
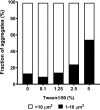
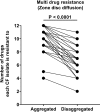
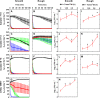
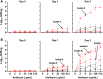
Pulmonary infection with the multidrug-resistant Mycobacterium abscessus complex (MABSC) is difficult to treat in individuals with cystic fibrosis (CF). MABSC grows as biofilm aggregates in CF patient lungs, which are known to have anaerobic niches. How aggregation and anoxic conditions affect antibiotic tolerance is not well understood. We sought to determine whether disaggregation and oxygen availability sensitize MABSC isolates to recommended antibiotics. We tested the susceptibilities of 33 isolates from 22 CF patients with MABSC infection and a reference strain to the following antibiotics: amikacin, azithromycin, cefoxitin, ciprofloxacin, clarithromycin, imipenem, kanamycin, linezolid, moxifloxacin, rifampin, tigecycline, and sulfamethoxazole-trimethoprim. Isolates were grown in Mueller-Hinton broth with and without the disaggregating detergent Tween 80 (5%). Time-kill curves at days 1 and 3 were generated for oxic and anoxic amikacin treatment in 4-fold dilutions ranging from 2 to 512 mg liter-1 Scanning electron microscopy was used to visualize the aggregation patterns, while confocal laser scanning microscopy and microrespirometry were used to visualize biofilm growth patterns. Disruption of MABSC aggregates increased susceptibility to amikacin, tigecycline, kanamycin, azithromycin, imipenem, cefoxitin, and clarithromycin (P < 0.05, n = 29 to 31). Oxygenation enhanced the killing of disaggregated MABSC isolates by amikacin (P < 0.05) by 1 to 6 log units when 2 to 512 mg liter-1 of amikacin was used. This study explains why current drug susceptibility testing results correlate poorly with treatment outcomes. The conditions achieved by oxic culturing of planktonic isolates in vitro do not resemble the hypoxic conditions in CF patient lungs. Biofilm disruption and increased O2 availability during antibiotic therapy may be new therapeutic strategies for chronic MABSC infection.
Keywords: Mycobacterium abscessus complex; antimicrobial resistance; biofilm; cystic fibrosis; oxygenation.
Copyright © 2020 American Society for Microbiology.
Figures



References
-
- Qvist T, Gilljam M, Jonsson B, Taylor-Robinson D, Jensen-Fangel S, Wang M, Svahn A, Kotz K, Hansson L, Hollsing A, Hansen CR, Finstad PL, Pressler T, Høiby N, Katzenstein TL, Scandinavian Cystic Fibrosis Study Consortium (SCFSC). 2015. Epidemiology of nontuberculous mycobacteria among patients with cystic fibrosis in Scandinavia. J Cyst Fibros 14:46–52. doi:10.1016/j.jcf.2014.08.002. - DOI - PMC - PubMed
-
- Qvist T, Taylor-Robinson D, Waldmann E, Olesen HV, Hansen CR, Mathiesen IH, Høiby N, Katzenstein TL, Smyth RL, Diggle PJ, Pressler T. 2016. Comparing the harmful effects of nontuberculous mycobacteria, and Gram negative bacteria on lung function in patients with cystic fibrosis. J Cyst Fibros 15:380–385. doi:10.1016/j.jcf.2015.09.007. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

