Novel Small Molecules Targeting the Intrinsically Disordered Structural Ensemble of α-Synuclein Protect Against Diverse α-Synuclein Mediated Dysfunctions
- PMID: 31740740
- PMCID: PMC6861283
- DOI: 10.1038/s41598-019-52598-4
Novel Small Molecules Targeting the Intrinsically Disordered Structural Ensemble of α-Synuclein Protect Against Diverse α-Synuclein Mediated Dysfunctions
Abstract
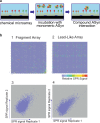
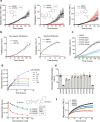
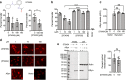
The over-expression and aggregation of α-synuclein (αSyn) are linked to the onset and pathology of Parkinson's disease. Native monomeric αSyn exists in an intrinsically disordered ensemble of interconverting conformations, which has made its therapeutic targeting by small molecules highly challenging. Nonetheless, here we successfully target the monomeric structural ensemble of αSyn and thereby identify novel drug-like small molecules that impact multiple pathogenic processes. Using a surface plasmon resonance high-throughput screen, in which monomeric αSyn is incubated with microchips arrayed with tethered compounds, we identified novel αSyn interacting drug-like compounds. Because these small molecules could impact a variety of αSyn forms present in the ensemble, we tested representative hits for impact on multiple αSyn malfunctions in vitro and in cells including aggregation and perturbation of vesicular dynamics. We thereby identified a compound that inhibits αSyn misfolding and is neuroprotective, multiple compounds that restore phagocytosis impaired by αSyn overexpression, and a compound blocking cellular transmission of αSyn. Our studies demonstrate that drug-like small molecules that interact with native αSyn can impact a variety of its pathological processes. Thus, targeting the intrinsically disordered ensemble of αSyn offers a unique approach to the development of small molecule research tools and therapeutics for Parkinson's disease.
Conflict of interest statement
Drs Agard and McConlogue are owners of Dainton Biosciences, LLC, which has rights to the described compounds. No other authors have competing interests.
Figures