The mutational footprints of cancer therapies
- PMID: 31740835
- PMCID: PMC6887544
- DOI: 10.1038/s41588-019-0525-5
The mutational footprints of cancer therapies
Abstract
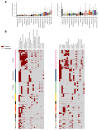
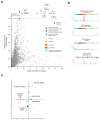
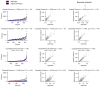
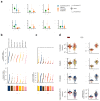
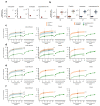
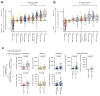
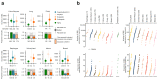
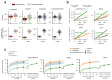
Some cancer therapies damage DNA and cause mutations in both cancerous and healthy cells. Therapy-induced mutations may underlie some of the long-term and late side effects of treatments, such as mental disabilities, organ toxicity and secondary neoplasms. Nevertheless, the burden of mutation contributed by different chemotherapies has not been explored. Here we identify the mutational signatures or footprints of six widely used anticancer therapies across more than 3,500 metastatic tumors originating from different organs. These include previously known and new mutational signatures generated by platinum-based drugs as well as a previously unknown signature of nucleoside metabolic inhibitors. Exploiting these mutational footprints, we estimate the contribution of different treatments to the mutation burden of tumors and their risk of contributing coding and potential driver mutations in the genome. The mutational footprints identified here allow for precise assessment of the mutational risk of different cancer therapies to understand their long-term side effects.
Conflict of interest statement
The authors declare no competing interests
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

