Cost-Effectiveness of Therapeutic Use of Safety-Engineered Syringes in Healthcare Facilities in India
- PMID: 31741306
- PMCID: PMC7250963
- DOI: 10.1007/s40258-019-00536-w
Cost-Effectiveness of Therapeutic Use of Safety-Engineered Syringes in Healthcare Facilities in India
Abstract
Background: Globally, 16 billion injections are administered each year of which 95% are for curative care. India contributes 25-30% of the global injection load. Over 63% of these injections are reportedly unsafe or deemed unnecessary.
Objectives: To assess the incremental cost per quality-adjusted life-year (QALY) gained with the introduction of safety-engineered syringes (SES) as compared to disposable syringes for therapeutic care in India.
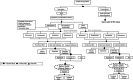
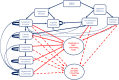
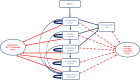
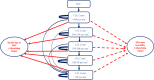
Methods: A decision tree was used to compute the volume of needle-stick injuries (NSIs) and reuse episodes among healthcare professionals and the patient population. Subsequently, three separate Markov models were used to compute lifetime costs and QALYs for individuals infected with hepatitis B virus (HBV), hepatitis C virus (HCV) and human immunodeficiency virus (HIV). Three SES were evaluated-reuse prevention syringe (RUP), sharp injury prevention (SIP) syringe, and syringes with features of both RUP and SIP. A lifetime study horizon starting from a base year of 2017 was considered appropriate to cover all costs and consequences comprehensively. A systematic review was undertaken to assess the SES effects in terms of reduction in NSIs and reuse episodes. These were then modelled in terms of reduction in transmission of blood-borne infections, life-years and QALYs gained. Future costs and consequences were discounted at the rate of 3%. Incremental cost per QALY gained was computed to assess the cost-effectiveness. A probabilistic sensitivity analysis was undertaken to account for parameter uncertainties.
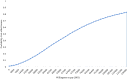
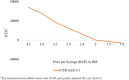
Results: The introduction of RUP, SIP and RUP + SIP syringes in India is estimated to incur an incremental cost of Indian National Rupee (INR) 61,028 (US$939), INR 7,768,215 (US$119,511) and INR 196,135 (US$3017) per QALY gained, respectively. A total of 96,296 HBV, 44,082 HCV and 5632 HIV deaths are estimated to be averted due to RUP in 20 years. RUP has an 84% probability to be cost-effective at a threshold of per capita gross domestic product (GDP). The RUP syringe can become cost saving at a unit price of INR 1.9. Similarly, SIP and RUP + SIP syringes can be cost-effective at a unit price of less than INR 1.2 and INR 5.9, respectively.
Conclusion: RUP syringes are estimated to be cost-effective in the Indian context. SIP and RUP + SIP syringes are not cost-effective at the current unit prices. Efforts should be made to bring down the price of SES to improve its cost-effectiveness.
Conflict of interest statement
Pankaj Bahuguna, Shankar Prinja, R.K. Dhiman, Madhumita Prem Kumar, Vineeta Sharma, A.K. Aggarwal and Rajesh Bhaskar declare they have no conflicts of interest.
Figures

Similar articles
-
Cost-Effectiveness of Temozolamide for Treatment of Glioblastoma Multiforme in India.JCO Glob Oncol. 2021 Jan;7:108-117. doi: 10.1200/GO.20.00288. JCO Glob Oncol. 2021. PMID: 33449801 Free PMC article.
-
Safety-Engineered Syringes: An Intervention to Decrease Hepatitis C Burden in Developing Countries-A Cost-Effectiveness Analysis From Egypt.Value Health Reg Issues. 2019 Sep;19:51-58. doi: 10.1016/j.vhri.2018.11.009. Epub 2019 Apr 16. Value Health Reg Issues. 2019. PMID: 31002984
-
Cost-effectiveness of FRAX®-based intervention thresholds for management of osteoporosis in Indian women: a Markov microsimulation model analysis.Osteoporos Int. 2025 Feb;36(2):311-322. doi: 10.1007/s00198-024-07328-6. Epub 2024 Dec 27. Osteoporos Int. 2025. PMID: 39730734
-
Is Reconstruction of Unstable Midfoot Charcot Neuroarthropathy Cost Effective from a US Payer's Perspective?Clin Orthop Relat Res. 2020 Dec;478(12):2869-2888. doi: 10.1097/CORR.0000000000001416. Clin Orthop Relat Res. 2020. PMID: 32694315 Free PMC article.
-
Multi-gene Pharmacogenomic Testing That Includes Decision-Support Tools to Guide Medication Selection for Major Depression: A Health Technology Assessment.Ont Health Technol Assess Ser. 2021 Aug 12;21(13):1-214. eCollection 2021. Ont Health Technol Assess Ser. 2021. PMID: 34484487 Free PMC article.
Cited by
-
Impact of Resistance Associated Substitutions and Predictors of Treatment Failure Following Direct-acting Antiviral Therapy in a Viral Hepatitis C Elimination Cohort.J Clin Exp Hepatol. 2025 Nov-Dec;15(6):102601. doi: 10.1016/j.jceh.2025.102601. Epub 2025 May 28. J Clin Exp Hepatol. 2025. PMID: 40611935
-
Economic Evaluation of Implementing a Rapid Point-of-Care Screening Test for the Identification of Hepatitis C Virus under National Viral Hepatitis Control Programme in Tamil Nadu, South India.J Glob Infect Dis. 2021 Aug 31;13(3):126-132. doi: 10.4103/jgid.jgid_394_20. eCollection 2021 Jul-Sep. J Glob Infect Dis. 2021. PMID: 34703152 Free PMC article.
-
Health-Related Quality of Life Among Liver Disorder Patients in Northern India.Indian J Community Med. 2022 Jan-Mar;47(1):76-81. doi: 10.4103/ijcm.ijcm_1033_21. Epub 2022 Mar 16. Indian J Community Med. 2022. PMID: 35368487 Free PMC article.
-
Cost-Effectiveness of Temozolamide for Treatment of Glioblastoma Multiforme in India.JCO Glob Oncol. 2021 Jan;7:108-117. doi: 10.1200/GO.20.00288. JCO Glob Oncol. 2021. PMID: 33449801 Free PMC article.
-
The state of cost-utility analysis in India: A systematic review.Perspect Clin Res. 2021 Oct-Dec;12(4):179-183. doi: 10.4103/picr.PICR_256_20. Epub 2021 Jul 12. Perspect Clin Res. 2021. PMID: 34760643 Free PMC article. Review.
References
-
- Atul K, Priya R, Thakur R, Gupta V, Kotwal J, Seth T. Injection practices in a metropolis of North India: perceptions, determinants and issues of safety. Indian J Med Sci. 2004;58(8):334–344. - PubMed
-
- Handbook on safe injection practices. In: Control NCFD, editor. New Delhi: GOI; 2014.
-
- Arora N. Injection practices in India. WHO South East Asia J Public Health. 2012;1(2):189–200. - PubMed
-
- Network, Safe Injection Global. Advocacy booklet. Switzerland: World Health Organization. 2011. p. 1–25
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

