Tumor-infiltrating lymphocytes from human prostate tumors reveal anti-tumor reactivity and potential for adoptive cell therapy
- PMID: 31741775
- PMCID: PMC6844325
- DOI: 10.1080/2162402X.2019.1672494
Tumor-infiltrating lymphocytes from human prostate tumors reveal anti-tumor reactivity and potential for adoptive cell therapy
Abstract
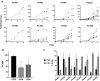
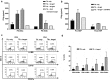
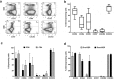
Advanced prostate cancer remains incurable and is the second leading cause of mortality in men. Immunotherapy based on the adoptive transfer of tumor-infiltrating lymphocytes (TIL) has demonstrated promising clinical results in patients with metastatic melanoma and lately also in other solid tumors. However, the ability to obtain TIL from patients with prostate cancer, considered poorly immunogenic, remains unknown. In this study, we investigate the feasibility of isolating and expanding TIL from primary prostate tumors. We collected tumor specimens from eight patients with diagnosed prostate adenocarcinoma undergoing radical prostatectomy and were able to successfully expand multiple autologous TIL cultures from all patients. Twenty-eight prostate-TIL cultures were further expanded using a standard rapid expansion procedure under Good Manufacturing Practice conditions. TIL cultures were phenotypically characterized for T cell subset composition, differentiation status and co-inhibitory/stimulatory markers such as PD-1, TIM-3, LAG-3, and CD28 and were found to have in general similarity to TIL obtained from patients with melanoma and lung carcinoma previously treated at our center. All analyzed TIL cultures were functional as determined by the capability to produce high level of IFNγ upon stimuli. Most importantly, co-culture assays of prostate-TIL with autologous tumors demonstrated anti-tumor reactivity. In conclusion, these findings demonstrate that functional and anti-tumor reactive TIL can be obtained, despite the immunosuppressive microenvironment of the cancer, thus this study supports the development of TIL therapy for prostate cancer patients.
Keywords: Tumor Infiltrating Lymphocyte (TIL); adoptive cell therapy (ACT); immunotherapy; primary prostate cancer cultures; prostate cancer (PCa).
© 2019 The Author(s). Published with license by Taylor & Francis Group, LLC.
Figures


Similar articles
-
Establishment of adoptive cell therapy with tumor infiltrating lymphocytes for non-small cell lung cancer patients.Cancer Immunol Immunother. 2018 Aug;67(8):1221-1230. doi: 10.1007/s00262-018-2174-4. Epub 2018 May 29. Cancer Immunol Immunother. 2018. PMID: 29845338 Free PMC article.
-
Bimodal ex vivo expansion of T cells from patients with head and neck squamous cell carcinoma: a prerequisite for adoptive cell transfer.Cytotherapy. 2011 Aug;13(7):822-34. doi: 10.3109/14653249.2011.563291. Epub 2011 Mar 24. Cytotherapy. 2011. PMID: 21428850
-
Expansion of tumor-infiltrating and marrow-infiltrating lymphocytes from pediatric malignant solid tumors.Cytotherapy. 2025 Jan;27(1):29-35. doi: 10.1016/j.jcyt.2024.08.002. Epub 2024 Aug 16. Cytotherapy. 2025. PMID: 39243253
-
White paper on adoptive cell therapy for cancer with tumor-infiltrating lymphocytes: a report of the CTEP subcommittee on adoptive cell therapy.Clin Cancer Res. 2011 Apr 1;17(7):1664-73. doi: 10.1158/1078-0432.CCR-10-2272. Epub 2011 Feb 15. Clin Cancer Res. 2011. PMID: 21325070 Review.
-
Tumor Infiltrating Lymphocyte (TIL) Therapy for Solid Tumor Treatment: Progressions and Challenges.Cancers (Basel). 2022 Aug 27;14(17):4160. doi: 10.3390/cancers14174160. Cancers (Basel). 2022. PMID: 36077696 Free PMC article. Review.
Cited by
-
Immunotherapy Vaccines for Prostate Cancer Treatment.Cancer Med. 2024 Oct;13(20):e70294. doi: 10.1002/cam4.70294. Cancer Med. 2024. PMID: 39463159 Free PMC article. Review.
-
Differential analysis of histopathological and genetic markers of cancer aggressiveness, and survival difference in EBV-positive and EBV-negative prostate carcinoma.Sci Rep. 2024 May 5;14(1):10315. doi: 10.1038/s41598-024-60538-0. Sci Rep. 2024. PMID: 38705879 Free PMC article.
-
Unlocking ferroptosis in prostate cancer - the road to novel therapies and imaging markers.Nat Rev Urol. 2024 Oct;21(10):615-637. doi: 10.1038/s41585-024-00869-9. Epub 2024 Apr 16. Nat Rev Urol. 2024. PMID: 38627553 Free PMC article. Review.
-
Has the Landscape of Immunotherapy for Prostate Cancer Changed? A Systematic Review and Post Hoc Analysis.Am J Mens Health. 2023 Mar-Apr;17(2):15579883231165140. doi: 10.1177/15579883231165140. Am J Mens Health. 2023. PMID: 37002863 Free PMC article.
-
Zinc finger C3H1 domain-containing protein (ZFC3H1) evaluates the prognosis and treatment of prostate adenocarcinoma (PRAD): A study based on TCGA data.Bioengineered. 2021 Dec;12(1):5504-5515. doi: 10.1080/21655979.2021.1965442. Bioengineered. 2021. PMID: 34514952 Free PMC article.
References
-
- Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, Mottet N, Schmid H-P, van der Kwast T, Wiegel T, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59:1–13. doi:10.1016/j.eururo.2010.10.039. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
