Evaluating quality of life tools in North American patients with erythropoietic protoporphyria and X-linked protoporphyria
- PMID: 31741822
- PMCID: PMC6850979
- DOI: 10.1002/jmd2.12052
Evaluating quality of life tools in North American patients with erythropoietic protoporphyria and X-linked protoporphyria
Abstract
Background: Erythropoietic protoporphyria (EPP) and X-linked Protoporphyria (XLP) are rare photodermatoses presenting with severe phototoxicity. Although anecdotally, providers who treat EPP patients acknowledge their life-altering effects, tools that fully capture their impact on quality of life (QoL) are lacking.
Methods: Adult patients with EPP/XLP were given four validated QoL tools: the Patient Reported Outcomes Measurement Information System 57 (PROMIS-57), the Hospital Anxiety and Depression Scale (HADS), the Illness Perception Questionnaire Revised (IPQR), and an EPP-Specific tool. All patients received the PROMIS-57 while the HADS, IPQR, and EPP-Specific tools were introduced at a later date. Associations between responses and clinical phenotypes were explored.
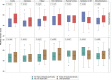
Results: Two hundred and two patients were included; 193 completed PROMIS-57, 104 completed IPQR, 103 completed HADS, and 107 completed the EPP-Specific tool. The IPQR showed that patients strongly believed EPP/XLP had a negative impact on their lives. Mean scores in anxiety and depression domains of both HADS and PROMIS-57 were normal; however, anxiety scores from HADS were borderline/abnormal in 20% of patients. The EPP-Specific tool revealed a decreased QoL in most patients. The PROMIS-57 showed that 21.8% of patients have clinically significant pain interference. Several tool domains correlated with measures of disease severity, most being from the PROMIS-57.
Conclusions: Impaired QoL is an important consequence of EPP/XLP. PROMIS-57 was most sensitive in evaluating impaired QoL in EPP/XLP. Further research is needed to compare the effectiveness of it for assessing response to treatment.
Keywords: PROMIS; erythropoietic proto; porphyria; quality of life.
© 2019 The Authors. Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
Hetanshi Naik reports consultancies with Alnylam. Jessica R. Overbey, Bruce Wang, Ashwani Singal, and Joseph Bloomer declare no conflict of interest. Robert J. Desnick reports consultancies from Alnylam Pharmaceuticals, Recordati Rare Diseases, and Mitsubishi Tanabe. Karl E. Anderson reports consultancies from Alnylam and grant (clinical trial) support from Alnylam and Mitsubishi Tanabe. D. Montgomery Bissell reports honoraria from Recordati and grant (clinical trial) support from Alnylam and Mitsubishi Tanabe. Herbert L. Bonkovsky reports consultancies form Alnylam, and Recordati Rare diseases and grant (clinical trial) support from Alnylam and Mitsubishi Tanabe. John D. Phillips reports consultancies from Alnylam, Recordati, and Agios and grant (clinical trial) support from Mitsubishi Tanabe. Manisha Balwani reports consultancies Alnylam, Recordati, and Mitsubishi Tanabe, and grant (clinical trial) support from Alnylam and Mitsubishi Tanabe.
Figures
References
-
- Anderson KE, Sassa S, Bishop DF, Desnick RJ. Disorders of heme biosynthesis: X‐linked sideroblastic anemia and the porphyrias In: CR S, AL B, Sly WS, Valle D, eds. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw Hill; 2014:71.
-
- Bonkovsky HL, Guo JT, Hou W, Li T, Narang T, Thapar M. Porphyrin and heme metabolism and the porphyrias. Compr Physiol. 2013;3:365‐401. - PubMed
-
- Poh‐Fitzpatrick MB. Porphyrias: photosensitivity and phototherapy. Methods Enzymol. 2000;319:485‐493. - PubMed
-
- Holme SA, Anstey AV, Finlay AY, Elder GH, Badminton MN. Erythropoietic protoporphyria in the U.K.: clinical features and effect on quality of life. Br J Dermatol. 2006;155:574‐581. - PubMed

