Lipofilling after breast conserving surgery: a comprehensive literature review investigating its oncologic safety
- PMID: 31741888
- PMCID: PMC6842766
- DOI: 10.21037/gs.2019.09.09
Lipofilling after breast conserving surgery: a comprehensive literature review investigating its oncologic safety
Abstract
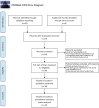
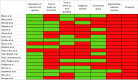
Lipofilling has regenerative properties used to improve deformities after breast conserving surgery. Our hypothesis is that there is inadequate data to ensure that lipofilling does not increase locoregional cancer recurrence after breast conserving surgery. A PRISMA comprehensive literature review was conducted of articles published prior to October 2019 investigating recurrence in patients who underwent lipofilling after breast conserving surgery. All forms of breast conserving surgery, fat grafting, and injection intervals were included. Patients undergoing mastectomy were excluded. Requirements to define lipofilling as "safe" included (I) a defined interval between resection and lipofilling; (II) a minimum follow-up period of 6 years from tumor resection; (III) a minimum follow-up period of 3 years from lipofilling; (IV) presence of a control group; (V) controls matched for ER/PR/Her-2; (VI) a sub-group analysis focusing on ER/PR/Her-2; (VII) adequate powering. Nineteen studies met inclusion criteria. The range in time from breast conserving surgery to fat injection was 0-76 months. The average time to follow-up after lipofilling was 23 days-60 months. Two studies had a sufficient follow-up time from both primary resection and from lipofilling. Seventeen of the nineteen studies specified the interval between resection and lipofilling, but there is currently no consensus regarding how soon lipofilling can be performed following BCS. Eight studies performed a subgroup analysis in cases of recurrence and found recurrence after lipofilling was associated with number of positive axillary nodes, intraepithelial neoplasia, high grade histology, Luminal A subtype, age <50, Ki-67 expression, and lipofilling within 3 months of primary resection. Of the eleven studies that included a comparison group, one matched patient for Her-2 and there was a statistically significant difference in Her-2 positive cancers in the study arms of two articles. Several studies deemed lipofilling "safe," two showed association of lipofilling and local recurrence, and most studies concluded that further research was needed. Insufficient and contradictory data exists to demonstrate the safety of lipofilling after breast conserving surgery. A multicentered, well designed study is needed to verify the safety of this practice.
Keywords: Breast conserving surgery; lipofilling; recurrence.
2019 Gland Surgery. All rights reserved.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures
Comment in
-
Lipofilling after breast conserving surgery: a plastic surgery perspective.Gland Surg. 2020 Jun;9(3):617-619. doi: 10.21037/gs.2020.04.02. Gland Surg. 2020. PMID: 32775249 Free PMC article. No abstract available.
-
Oncological safety of lipofilling after breast conserving surgery.Gland Surg. 2020 Jun;9(3):620-621. doi: 10.21037/gs.2020.03.23. Gland Surg. 2020. PMID: 32775250 Free PMC article. No abstract available.
References
-
- Lamszus K, Jin L, Fuchs A, et al. Scatter factor stimulates tumor growth and tumor angiogenesis in human breast cancers in the mammary fat pads of nude mice. Lab Invest 1997;76:339-53. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
