Single-molecule studies of classical and desmosomal cadherin adhesion
- PMID: 31742239
- PMCID: PMC6859941
- DOI: 10.1016/j.cobme.2019.08.006
Single-molecule studies of classical and desmosomal cadherin adhesion
Abstract
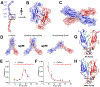
Classical cadherin and desmosomal cadherin cell-cell adhesion proteins play essential roles in tissue morphogenesis and in maintaining tissue integrity. Deficiencies in cadherin adhesion are hallmarks of diseases like cancers, skin diseases and cardiomyopathies. Structural studies and single molecule biophysical measurements have revealed critical similarities and surprising differences between these key adhesion proteins. This review summarizes our current understanding of the biophysics of classical and desmosomal cadherin adhesion and the molecular basis for their cross-talk. We focus on recent single molecule measurements, highlight key insights into the adhesion of cadherin extracellular regions and their relation to associated diseases, and identify major open questions in this exciting area of research.
Conflict of interest statement
Conflict of Interest: The authors declare no conflict of interest.
Figures
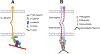
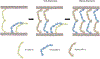
References
-
- Najor NA, Desmosomes in Human Disease. Annual Review of Pathology: Mechanisms of Disease, 2018. 13(1): p. 51–70. - PubMed
-
- Priest AV, Shafraz O, and Sivasankar S, Biophysical basis of cadherin mediated cell-cell adhesion. Experimental Cell Research, 2017. 358(1): p. 10–13. - PubMed
-
- Boggon TJ, Murray J, Chappuis-Flament S, Wong E, Gumbiner BM, and Shapiro L, C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science, 2002. 296(5571): p. 1308–1313. - PubMed
