Synthesis and Characterization of Long-Acting Darunavir Prodrugs
- PMID: 31742407
- PMCID: PMC7295016
- DOI: 10.1021/acs.molpharmaceut.9b00871
Synthesis and Characterization of Long-Acting Darunavir Prodrugs
Abstract
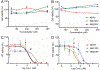
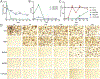
Antiretroviral therapy (ART) has improved the quality of life in patients infected with HIV-1. However, complete viral suppression within anatomical compartments remains unattainable. This is complicated by adverse side effects and poor adherence to lifelong therapy leading to the emergence of viral drug resistance. Thus, there is an immediate need for cellular and tissue-targeted long-acting (LA) ART formulations. Herein, we describe two LA prodrug formulations of darunavir (DRV), a potent antiretroviral protease inhibitor. Two classes of DRV prodrugs, M1DRV and M2DRV, were synthesized as lipophilic and hydrophobic prodrugs and stabilized into aqueous suspensions designated NM1DRV and NM2DRV. The formulations demonstrated enhanced intracellular prodrug levels with sustained drug retention and antiretroviral activities for 15 and 30 days compared to native DRV formulation in human monocyte-derived macrophages. Pharmacokinetics tests of NM1DRV and NM2DRV administered to mice demonstrated sustained drug levels in blood and tissues for 30 days. These data, taken together, support the idea that LA DRV with sustained antiretroviral responses through prodrug nanoformulations is achievable.
Keywords: LASER ART; darunavir; long acting; prodrugs; protease inhibitors.
Figures
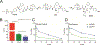

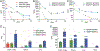
References
-
- Giacomelli A, Riva A, Falvella FS, Oreni ML, Cattaneo D, Cheli S, et al. Clinical and genetic factors associated with increased risk of severe liver toxicity in a monocentric cohort of HIV positive patients receiving nevirapine-based antiretroviral therapy. BMC infectious diseases. 2018;18(1):556. - PMC - PubMed
-
- Fogarty L, Roter D, Larson S, Burke J, Gillespie J, Levy R. Patient adherence to HIV medication regimens: a review of published and abstract reports. Patient Educ Couns. 2002;46(2):93–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS036126/NS/NINDS NIH HHS/United States
- R01 AI145542/AI/NIAID NIH HHS/United States
- P01 DA028555/DA/NIDA NIH HHS/United States
- P30 MH062261/MH/NIMH NIH HHS/United States
- R56 AI138613/AI/NIAID NIH HHS/United States
- P01 MH064570/MH/NIMH NIH HHS/United States
- R01 MH104147/MH/NIMH NIH HHS/United States
- R01 AG043540/AG/NIA NIH HHS/United States
- P30 AI078498/AI/NIAID NIH HHS/United States
- R01 NS034239/NS/NINDS NIH HHS/United States
- R01 AI158160/AI/NIAID NIH HHS/United States
- P01 NS031492/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources

