Pharmacological interventions for painful sickle cell vaso-occlusive crises in adults
- PMID: 31742673
- PMCID: PMC6863096
- DOI: 10.1002/14651858.CD012187.pub2
Pharmacological interventions for painful sickle cell vaso-occlusive crises in adults
Abstract
Background: Sickle cell disease (SCD) is a group of inherited disorders of haemoglobin (Hb) structure in a person who has inherited two mutant globin genes (one from each parent), at least one of which is always the sickle mutation. It is estimated that between 5% and 7% of the world's population are carriers of the mutant Hb gene, and SCD is the most commonly inherited blood disorder. SCD is characterized by distorted sickle-shaped red blood cells. Manifestations of the disease are attributed to either haemolysis (premature red cell destruction) or vaso-occlusion (obstruction of blood flow, the most common manifestation). Shortened lifespans are attributable to serious comorbidities associated with the disease, including renal failure, acute cholecystitis, pulmonary hypertension, aplastic crisis, pulmonary embolus, stroke, acute chest syndrome, and sepsis. Vaso-occlusion can lead to an acute, painful crisis (sickle cell crisis, vaso-occlusive crisis (VOC) or vaso-occlusive episode). Pain is most often reported in the joints, extremities, back or chest, but it can occur anywhere and can last for several days or weeks. The bone and muscle pain experienced during a sickle cell crisis is both acute and recurrent. Key pharmacological treatments for VOC include opioid analgesics, non-opioid analgesics, and combinations of drugs. Non-pharmacological approaches, such as relaxation, hypnosis, heat, ice and acupuncture, have been used in conjunction to rehydrating the patient and reduce the sickling process.
Objectives: To assess the analgesic efficacy and adverse events of pharmacological interventions to treat acute painful sickle cell vaso-occlusive crises in adults, in any setting.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online, MEDLINE via Ovid, Embase via Ovid and LILACS, from inception to September 2019. We also searched the reference lists of retrieved studies and reviews, and searched online clinical trial registries.
Selection criteria: Randomized, controlled, double-blind trials of pharmacological interventions, of any dose and by any route, compared to placebo or any active comparator, for the treatment (not prevention) of painful sickle cell VOC in adults.
Data collection and analysis: Three review authors independently assessed studies for eligibility. We planned to use dichotomous data to calculate risk ratio (RR) and number needed to treat for one additional event, using standard methods. Our primary outcomes were participant-reported pain relief of 50%, or 30%, or greater; Patient Global Impression of Change (PGIC) very much improved, or much or very much improved. Our secondary outcomes included adverse events, serious adverse events, and withdrawals due to adverse events. We assessed GRADE and created three 'Summary of findings' tables.
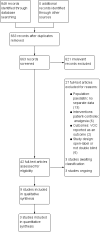
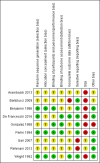
Main results: We included nine studies with data for 638 VOC events and 594 participants aged 17 to 42 years with SCD presenting to a hospital emergency department in a painful VOC. Three studies investigated a non-steroidal anti-inflammatory drug (NSAID) compared to placebo. One study compared an opioid with a placebo, two studies compared an opioid with an active comparator, two studies compared an anticoagulant with a placebo, and one study compared a combination of three drugs with a combination of four drugs. Risk of bias across the nine studies varied. Studies were primarily at an unclear risk of selection, performance, and detection bias. Studies were primarily at a high risk of bias for size with fewer than 50 participants per treatment arm; two studies had 50 to 199 participants per treatment arm (unclear risk). Non-steroidal anti-inflammatory drugs (NSAID) compared with placebo No data were reported regarding participant-reported pain relief of 50% or 30% or greater. The efficacy was uncertain regarding PGIC very much improved, and PGIC much or very much improved (no difference; 1 study, 21 participants; very low-quality evidence). Very low-quality, uncertain results suggested similar rates of adverse events across both the NSAIDs group (16/45 adverse events, 1/56 serious adverse events, and 1/56 withdrawal due to adverse events) and the placebo group (19/45 adverse events, 2/56 serious adverse events, and 1/56 withdrawal due to adverse events). Opioids compared with placebo No data were reported regarding participant-reported pain relief of 50% or 30%, PGIC, or adverse events (any adverse event, serious adverse events, and withdrawals due to adverse events). Opioids compared with active comparator No data were reported regarding participant-reported pain relief of 50% or 30% or greater. The results were uncertain regarding PGIC very much improved (33% of the opioids group versus 19% of the placebo group). No data were reported regarding PGIC much or very much improved. Very low-quality, uncertain results suggested similar rates of adverse events across both the opioids group (9/66 adverse events, and 0/66 serious adverse events) and the placebo group (7/64 adverse events, 0/66 serious adverse events). No data were reported regarding withdrawal due to adverse events. Quality of the evidence We downgraded the quality of the evidence by three levels to very low-quality because there are too few data to have confidence in results (e.g. too few participants per treatment arm). Where no data were reported for an outcome, we had no evidence to support or refute (quality of the evidence is unknown).
Authors' conclusions: This review identified only nine studies, with insufficient data for all pharmacological interventions for analysis. The available evidence is very uncertain regarding the efficacy or harm from pharmacological interventions used to treat pain related to sickle cell VOC in adults. This area could benefit most from more high quality, certain evidence, as well as the establishment of suitable registries which record interventions and outcomes for this group of people.
Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
TC: none known.
IH: none known.
SB is an internist specializing in basic and clinical haematology with an emphasis on the management of adult and children with sickle cell disease and pain. SB has been in the Speakers Bureau of Novartis with an emphasis on Deferasirox (both Exjade and Jadenu formulations) in September 2016.
BJ: none known.
PW undertakes work for GSK under the auspices of his company Oxford Systematic Review Services. The work is related to over‐the‐counter analgesics.
The protocol for this review was identified in a 2019 audit as not meeting the current definition of the Cochrane Commercial Sponsorship policy. At the time of its publication it was compliant with the interpretation of the existing policy. A new author team fully compliant with the 2014 policy completed the review. As with all reviews, new and updated, at update this review will be revised according to 2020 policy update.
Figures





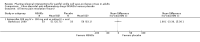
Update of
References
References to studies included in this review
Arambasik 2013 {published data only}
-
- Arambasik J, Griffin P, Zimmerman J, Beeson M, McMullen MJ, Griffin G, et al. The use of low dose ketamine and hydromorphone for patients in sickle cell crisis. Academic Emergency Medicine 2013;1:S149.
Bartolucci 2009 {published data only}
Benjamin 1986 {published data only}
-
- Benjamin LJ, Berkowitz LR, Orringer E. A collaborative, double‐blind randomized study of cetiedil citrate in sickle cell crisis. Blood 1986;67(5):1442‐7. [online ISSN 1528‐0020] - PubMed
De Franceschi 2016 {published data only}
-
- Franceschi L, Mura P, Schweiger V, Venacto E, Quaglia FM, Delmonte L, et al. Fentanyl buccal tablet: a new breakthrough pain medication in early management of severe vaso‐occlusive crisis in sickle cell disease. Pain Practice 2016;16(3):680‐7. - PubMed
Gonzalez 1988 {published data only}
Perlin 1994 {published data only}
-
- Perlin E. Intravenous ketorolac tromethamine enhances pain control in sickle cell vaso‐occlusive crisis [abstract]. 18th Annual Meeting of the National Sickle Cell Disease Program; Philadelphia, PA, USA. (May 24‐26) 1993; Vol. 65a.
-
- Perlin E, Finke H, Castro O, Rana S, Pittman J, Burt R, et al. Enhancement of pain control with ketorolac tromethamine in patients with sickle cell vaso‐occlusive crisis. American Journal of Hematology 1994;46(1):43‐7. - PubMed
Qari 2007 {published data only}
-
- Qari MH, Aljaouni SK, Alardawi MS, Fatani H, Alsayes FM, Zografos P, et al. Reduction of painful vaso‐occlusive crisis of sickle cell anaemia by tinzaparin in a double‐blind randomized trial. Thrombosis and Haemostasis 2007;98(2):392‐6. - PubMed
Rehmani 2013 {published data only}
-
- Rehmani R. A randomized controlled trial of paracetamol versus morphine for the treatment of acute painful crisis of sickle cell disease. Academic Emergency Medicine 2013;20(5 Suppl 1):S149. [DOI: 10.1111/acem.12115] - DOI
Wright 1992 {published data only}
-
- Wright SW, Norris RL, Mitchell TR. Ketorolac for sickle cell vaso‐occlusive crisis pain in the emergency department: lack of a narcotic‐sparing effect. Annals of Emergency Medicine 1992;21(8):925‐8. - PubMed
References to studies excluded from this review
Al‐Jam'a 1999 {published data only}
-
- Al‐Jam'a AH, Al‐Dabbous IA, Rafiq MS, Al‐Khatti A, Al‐Salem AH, Al‐Bahrna A, et al. Isoxsuprine in the treatment of sickle cell painful crises: a double‐blind comparative study with narcotic analgesic. Annals of Saudi Medicine 1999;19(2):97‐100. - PubMed
Ataga 2018 {published data only}
-
- Ataga KI, Hoppe CC, Ware RE, Howard J, Tonda M, Ganju J, et al. Novel trial design to evaluate oral voxelotor for the treatment of sick cell disease: The phase 3 hemoglobin oxygen affinity modulation to inhibit sickle hemoglobin polymerization (HOPE) trial. HemaSphere 2018;2(Suppl 2):670.
Ballas 2010 {published data only}
-
- Ballas SK, Bauserman RL, McCarthy WF, Castro OL, Smith WR, Waclawiw MA. Hydroxyurea and acute painful crises in sickle cell anemia: effects of hospital length of stay and opioid utilization during hospitalisation, outpatient acute care contacts, and at home. Journal of Pain and Symptom Management 2010;40(6):870‐82. - PMC - PubMed
Cho 2016 {published data only}
Dampier 2010 {published data only}
-
- Dampier C, Smith WR, Wager C, Bell M, Eckman JR, Hsu LL, et al. Initial experience with the IMPROVE trial – a phase III analgesic trial for hospitalized sickle cell painful episodes. Blood 2010;116(21):2667.
Dampier 2013 {published data only}
-
- Dampier CD, Smith WR, Wager CG, Kim HY, Bell MC, Miller ST, et al. IMPROVE trial: a randomized controlled trial of patient‐controlled analgesia for sickle cell painful episodes: rationale, design challenges, initial experience, and recommendations for future studies. Clinical Trials 2013;10(2):319‐31. [DOI: 10.1177/1740774513475850] - DOI - PMC - PubMed
Desai 2013 {published data only}
Euctr 2008 {published data only}
-
- Euctr GB. An evaluation of the effectiveness of ibuprofen and morphine for acute pain in sickle cell disease ‐ ibuprofen and morphine for acute sickle cell pain. http://www.who.int/trialsearch/Trial2.aspx?TriallD=EUCTR2008‐0068‐24‐GB 2008.
Euctr 2011 {published data only}
-
- Euctr IE. Sickle cell anaemia is an inherited blood disorder which results in abnormal sickle shaped red blood cells which do not fit well through small blood vessels. These blockages prevention oxygen (in blood) from reaching different parts of the body resulting in painful crisis. This study will compared the effectiveness of two types of pain medication, one given through a vein and one squirted up the nose. http://www.who.int/trialsearch/Trial2.aspx?TriallD=EUCTR2011‐005161‐20‐IE 2011.
Gonzalez 1991 {published data only}
-
- Gonzalez ER, Bahal N, Hansen LA, Ware D, Bull DS, Ornato JP, et al. Intermittent injection vs patient‐controlled analgesia for sickle cell crisis pain. Comparison in patients in the emergency department. Archives of Internal Medicine 1991;151(7):1373‐8. - PubMed
Isrctn 2006 {published data only}
-
- Isrctn. Patient controlled analgesia (PCA) versus continuous infusion (CI) of morphine during vaso‐occlusive crisis in sickle cell disease (SCD): a randomised controlled trial. http://www.who.int/trialsearch/Trial2.aspx?TriallD=ISRCTN74336585 2006. - PubMed
Isrctn 2009 {published data only}
-
- Isrctn. Use of intravenous paracetamol in combination with morphine in sickle cell disease children with vaso‐occlusive crisis. http://www.who.int/trialsearch/Trial2.aspx?TriallD=ISRCTN34491330 2009.
Morris 2009 {published data only}
Nct 2019a {published data only}
-
- Nct. Nebulized sub‐dissociative dose ketamine at three different dosing regimens for treating acute pain in the pediatric ED. https://clinicaltrials.gov/show/NCT03950817 2019.
Nct 2019b {published data only}
-
- Nct. Ketamine infusion for sickle cell pain crisis. https://clinicaltrials.gov/show/NCT04005209 2019.
Niihara 2018 {published data only}
-
- Niihara Y, Stark CW, Razon R, Tran LT, Becerra JM, Panosyan EH, Lasky JL. Consistent benefit of L‐glutamine observed across patients with low, medium, and high number of crises reported in the year prior to screening‐analysis from the phase 3 study of L‐glutamine in sickle cell anemia. Blood 2018;132(Suppl 1):1065.
Orringer 2001 {published data only}
-
- Orringer EP, Casella JF, Ataga KI, Koshy M, Adams‐Graves P, Luchtman‐Jones L, et al. Purified poloxamer 188 for treatment of acute vaso‐occlusive crisis of sickle cell disease: a randomized controlled trial. Journal of the American Medical Association 2001;286(17):2099‐106. - PubMed
Pactr 2018 {published data only}
-
- Pactr. A comparative analysis of parenteral opioids in the management of acute sickle cell crisis in an urban primary care centre (Korle bu Polyclinic) in Accra. http://www/who.int/trialsearch/Trial2.aspx?TriallD=PACTR201812917283565 2018.
Puri 2019 {published data only}
-
- Puri L, Anghelescu D, Hankins J, Nottage K, Wang W, Kang G, et al. Gabapentin for pain in sickle cell disease: Results of a randomized phase II clinical trial. Pediatric Blood and Cancer 2019;66:S7.
Rousseau 2015 {published data only}
-
- Rousseau V, Arriuberge C, Chabaud S, Launay V, Thouvenin S, Tourniaire B, et al. Efficacy and tolerance of lidocaine 5% patches in neuropathic pain and vaso‐occlusive sickle‐cell crises pain in children: a prospective multi‐centre clinical study. Pediatric Blood and Cancer 2015;62:S384. - PubMed
Sandoval 2013 {published data only}
-
- Sandoval M, Coleman P, Govani R, Siddiqui S, Todd KH. Pilot study of human recombinant hyaluronidase‐enhance subcutaneous hydration and opioid administration for sickle cell disease acute pain episodes. Journal of Pain & Palliative Care Pharmacotherapy 2013;27(1):10‐8. - PubMed
Shah 2019 {published data only}
-
- Shah N, Boccia R, Kraft WK, Hardesty BM, Paulose J, Lain D, et al. Pro3 successor study: treatment and health care resource utilization by sickle cell patients who participated in the sustain study in the United States. Value in Health 2019;22(Suppl 2):S335.
Smith 2011 {published data only}
Tanabe 2018 {published data only}
Telen 2015 {published data only}
-
- Telen MJ, Wun T, McCavit TL, Castro LM, Krishnamurti L, Lanzkron S. GMI 1070: reduction in time to resolution of vaso‐occlusive crisis and decreased opioid use in a prospective, randomized, multi‐centre double blind, adaptive phase 2 study in sickle cell disease. Blood 2013; Vol. 122:21.
Uzun 2010 {published data only}
-
- Uzun B, Kekec Z, Gurkan E. Efficacy of tramadol vs meperidine in vasoocclusive sickle cell crisis. American Journal of Emergency Medicine 2010;28(4):445‐9. - PubMed
van Beers 2007 {published data only}
-
- Beers EJ, Tuijn CF, Nieuwkerk PT, Friederich PW, Vranken JH, Biemond BJ. Patient‐controlled analgesia versus continuous infusion of morphine during vaso‐occlusive crisis in sickle cell disease, a randomized controlled trial. American Journal of Hematology 2007;82(11):955‐60. - PubMed
References to studies awaiting assessment
De Castro 2013 {published data only}
-
- Castro LM, Wun T, Lanzkron S, Smith WR, Hassell KL, Kutlar A, et al. Effects of GMI‐1070, a pan‐selection inhibitor, on pain intensity and opioid utilization in sickle cell disease. Blood 2013;122(21):775.
Perlin 1988 {published data only}
-
- Perlin E, Finke H, Castro O, Bang KM, Rana S, Taylor R, et al. Treatment of sickle cell pain crisis. A clinical trial of diflunisal. Clinical Trials Journal 1988;25(4):254‐64.
Teuscher 1989 {published data only}
-
- Teuscher T, Ahe CW, Balillod P, Holzer B. Double‐blind randomised clinical trial of pentoxiphyllin in vaso‐occlusive sickle cell crisis. Tropical & Geographical Medicine 1989;41(4):320‐5. - PubMed
References to ongoing studies
IRCT2016072511956N6 {published data only}
-
- IRCT2016072511956N6. To relieve crisis pain in sickle cell anemia patients. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2016072511956N6 (accessed September 2019).
NCT03431285 {published data only}
-
- NCT03431285. Ketamine for acute painful crisis in sickle cell disease patients. clinicaltrials.gov/ct2/show/NCT03431285 (first received 13 February 2018).
NCT03978156 {published data only}
-
- NCT03978156. Dronabinol for pain and inflammation in adults living with sickle cell disease. https://clinicaltrials.gov/show/NCT03978156 (accessed September 2019) 2019.
Additional references
Al Hajeri 2007
Ballas 2005
Ballas 2007
De Ceulaer 1982
Dechartres 2013
Dechartres 2014
Diallo 2002
-
- Diallo D, Tchernia G. Sickle cell disease in Africa. Current Opinion in Hematology 2002;9(2):111‐6. [ISSN: 1065‐6251] - PubMed
Dunlop 2014
Dworkin 2008
Gaston 1986
Gillis 2012
-
- Gillis VL, Senthinathan A, Dzingina M, Chamberlain K, Banks E, Baker MR, Longson D. Management of an acute painful sickle cell episode in hospital: summary of NICE guidance. BMJ 2012;344:e4063. - PubMed
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2013a
Guyatt 2013b
Herrick 1910
-
- Herrick J. Peculiar elongated and sickle‐shaped red blood corpuscles in a case of severe anemia. Archives of Internal Medicine 1910;5(5):517‐21. [DOI: ]
Higgins 2011a
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoffman 2010
Hussein 2015
L'Abbé 1987
Lane 2001
-
- Lane PA, Nuss R, Ambruso D. Hematologic disorders. In: Hay WW, Hayward AR, Levin MJ, Sondheimer JM editor(s). Current Pediatric Diagnosis and Treatment. 15th Edition. New York (NY): McGraw‐Hill Companies Inc, 2001:756‐9.
Lutz 2015
Martí‐Carvajal 2009
McQuay 1998
-
- McQuay H, Moore R. An Evidence‐based Resource for Pain Relief. Oxford: Oxford University Press, 1998. [ISBN: 0‐19‐263048‐2]
Moher 2009
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle (WA): IASP Press, 2008:15‐24. [ISBN: 978–0–931092–69–5]
Moore 2010a
Moore 2010b
-
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374‐9. [DOI: 10.1136/ard.2009.107805] - DOI - PMC - PubMed
Moore 2010c
Moore 2013
National Screening Committee 2006
-
- National Screening Committee for Sickle Cell Disease and Thalassaemia. NHS Sickle Cell and Thalassaemia Screening Programme, 2006. www.gov.uk/topic/population‐screening‐programmes/sickle‐cell‐thalassaemia (accessed 7 September 2015).
NICE 2015
-
- National Institute of Health and Care Excellence (NICE). Guidance – sickle cell acute painful episode, 2015. (accessed 7 September 2015).
NIH 2014
-
- National Institutes of Health. Evidence‐based management of sickle cell disease: expert panel report, 2014. www.nhlbi.nih.gov/guidelines (accessed 15 August 2017).
NIH 2015
-
- National Heart, Lung and Blood Institute. Sickle cell disease, 2015. www.nhlbi.nih.gov/health/health‐topics/topics/sca/atrisk (accessed 12 October 2015).
Nüesch 2010
O'Brien 2010
Okomo 2015
Okpala 2002
Piel 2013
Platt 1994
Quinn 2010
Rees 2003
Review Manager 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosenblum 2008
Sasongko 2013
Serjeant 1994
-
- Serjeant GR. The geography of sickle‐cell disease: opportunity for understanding its diversity. Annals of Saudi Medicine 1994;14(3):237‐46. [PUBMED: 17586900] - PubMed
Stuart 2004
Tanabe 2012
-
- Tanabe P, Hafner J, Martinovich Z, Artz N. Adult emergency department patients with sickle cell pain crisis: results from a quality improvement learning collaborative model to improve analgesic management. Academic Emergency Medicine 2012;9(4):430‐8. [DOI: 10.1111/j.1553-2712.2012.01330.x] - DOI - PMC - PubMed
Telfer 2014
Thienhaus 2002
-
- Thienhaus O, Cole BE. Classification of pain. In: Weiner RS editor(s). Pain management: a practical guide for clinicians. 6th Edition. New York (NY): CRC Press, 2002.
Thorlund 2011
Trescot 2008
-
- Trescot M, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain Physician Journal 2008;11(2S):S133‐53. [ISSN: 1533‐3159; PUBMED: 18443637] - PubMed
Waldrop 1995
Weatherall 2001
Weatherall 2006
-
- Weatherall D, Akinyanju O, Fucharoen S, Olivieri N, Musgrove P. Inherited disorders of hemoglobin. Disease Control Priorities in Developing Countries. 2nd Edition. Washington (DC): World Bank and Oxford University Press, 2006:663‐80.
WHO 2010
-
- World Health Organization. Sickle‐cell disease: a strategy for the WHO African region 2010. www.afro.who.int/en/sixtieth‐session.html (accessed 19 October 2015).
WHO 2011
-
- World Health Organization. Sickle‐cell disease and other haemoglobin disorders: fact sheet N°308. Geneva: World Health Organization, 2011.
Wiffen 2005
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

