Cobalt(III)-Catalyzed C-H Amidation of Dehydroalanine for the Site-Selective Structural Diversification of Thiostrepton
- PMID: 31742803
- PMCID: PMC6940514
- DOI: 10.1002/anie.201911886
Cobalt(III)-Catalyzed C-H Amidation of Dehydroalanine for the Site-Selective Structural Diversification of Thiostrepton
Abstract
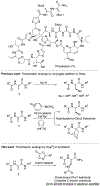
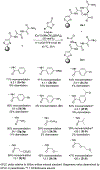
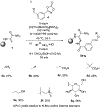
Thiostrepton is a potent antibiotic against a broad range of Gram-positive bacteria, but its medical applications have been limited by its poor aqueous solubility. In this work, the first C(sp2 )-H amidation of dehydroalanine (Dha) residues was applied to the site selective modification of thiostrepton to prepare a variety of derivatives. Unlike all prior methods for the modification of thiostrepton, the alkene framework of the Dha residue is preserved and with complete selectivity for the Z-stereoisomer. Additionally, an aldehyde group was introduced by C-H amidation, enabling oxime ligation for the installation of an even greater range of functionality. The thiostrepton derivatives generally maintain antimicrobial activity, and importantly, eight of the derivatives displayed improved aqueous solubility (up to 28-fold), thereby addressing a key shortcoming of this antibiotic. The exceptional functional group compatibility and site selectivity of CoIII -catalyzed C(sp2 )-H Dha amidation suggests that this approach could be generalized to other natural products and biopolymers containing Dha residues.
Keywords: C−H activation; antibiotics; chemoselectivity; cobalt; natural products.
© 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

References
-
- For leading references on biosynthetic methods to access thiostrepton derivatives, see:Wang X, Lin Z, Bai X, Tao J, Liu W, Org. Chem. Front 2019, 6, 1194–1199.
-
- For leading references on the chemical functionalization of Dha residues, see: (a) Bogart JW, Bowers AA, Org. Biomol. Chem 2019, 17, 3653–3669. - PMC - PubMed
- Dadová J, Galan SRG, Davis BG, Curr. Opin. Chem. Biol 2018, 46, 71–81. - PubMed
- Cox CL, Tietz JI, Sokolowski K, Melby JO, Doroghazi JR, Mitchell DA, ACS Chem. Biol 2014, 9, 2014–2022. - PMC - PubMed
- Naidu BN, Li W, Sorenson ME, Connolly TP, Wichtowski JA, Zhang Y, Kim OK, Matiskella JD, Lam KS, Bronson JJ, Ueda Y, Tetrahedron Lett. 2004, 45, 1059–1063. - PubMed
- (e) Zhu Y, van der Donk W, Org. Lett 2001, 38, 1189–1192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

