Efficacy and safety of dapagliflozin in Japanese patients with inadequately controlled type 1 diabetes (DEPICT-5): 52-week results from a randomized, open-label, phase III clinical trial
- PMID: 31742898
- PMCID: PMC7078973
- DOI: 10.1111/dom.13922
Efficacy and safety of dapagliflozin in Japanese patients with inadequately controlled type 1 diabetes (DEPICT-5): 52-week results from a randomized, open-label, phase III clinical trial
Abstract
Aims: To investigate the safety and tolerability of 5 and 10 mg dapagliflozin added to insulin therapy over 52 weeks in Japanese patients with inadequately controlled type 1 diabetes mellitus (T1DM).
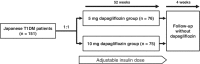
Materials and methods: This randomized, open-label, parallel-group, multicentre phase III clinical trial was conducted from October 26, 2015 to June 15, 2017. The primary endpoint was the occurrence of adverse events such as hypoglycaemia and diabetic ketoacidosis. Secondary endpoints included changes in glycaemic parameters, total daily insulin dosage and body weight over time. The efficacy of dapagliflozin in patients stratified by body mass index (BMI) <25.0 and ≥25.0 kg/m2 was evaluated in a subgroup analysis.
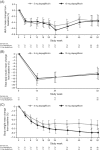
Results: In total, 151 patients received 5 mg (n = 76) or 10 mg (n = 75) dapagliflozin once daily for 52 weeks. Adverse events were observed in 88.2% and 73.3% of patients in the 5 and 10 mg dapagliflozin groups, respectively. Severe hypoglycaemia was reported in 2.6% (n = 2) and 6.7% (n = 5) of patients, and diabetic ketoacidosis in 2.6% (n = 2) and 1.3% (n = 1) of patients in the 5 and 10 mg dapagliflozin groups, respectively. The adjusted mean (95% confidence interval) changes in glycated haemoglobin at week 52 were -0.33% (-0.50, -0.15) and -0.36% (-0.53, -0.18) in the 5 and 10 mg dapagliflozin groups, respectively. There were no differences in efficacy parameters when stratified by BMI.
Conclusions: This study demonstrated the long-term safety and tolerability of dapagliflozin added to insulin therapy in Japanese patients with inadequately controlled T1DM.
© 2019 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
E.A. has participated on advisory panels for Alcon, Astellas Pharma, AstraZeneca, Eli Lilly, Kowa Pharmaceutical, Nippon Boehringer Ingelheim, Novo Nordisk Pharma, Sanofi, and Terumo Corporation; has received honoraria for lectures from Astellas Pharma, MSD, Ono Pharmaceutical, Novo Nordisk Pharma, and Sanofi; and has received scholarship grants from Astellas Pharma, Daiichi Sankyo, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Novo Nordisk Pharma, Ono Pharmaceutical, Sanofi, Shionogi, Sumitomo Dainippon Pharma, and Takeda Pharmaceutical. H.W. reports acting as an advisory board member for Novo Nordisk and as a speaker for Astellas Pharma, Sanofi, Mitsubishi Tanabe Pharma, Novo Nordisk, Kowa Pharmaceutical, AstraZeneca, Takeda Pharmaceutical, Novartis, Nippon Boehringer Ingelheim, Merck Sharp & Dohme, Sumitomo Dainippon Pharma, Eli Lilly Japan, Sanwa Kagaku Kenkyusho, Ono Pharmaceutical, Kissei Pharmaceutical, and FUJIFILM Pharma; and receiving grants from Astellas Pharma, Sanofi, Mitsubishi Tanabe Pharma, Novo Nordisk Pharma, AstraZeneca, Takeda Pharmaceutical, Novartis Pharma, Nippon Boehringer Ingelheim, Merck Sharp & Dohme, Sumitomo Dainippon Pharma, Eli Lilly Japan, Ono Pharmaceutical, Kyowa Kirin, Daiichi Sankyo, Terumo, Pfizer Japan, Mochida Pharmaceutical, Taisho Toyama Pharmaceutical, Johnson & Johnson, and Kowa. Y.U. has received grants and honoraria from AstraZeneca K.K. and holds a position as a board member for AstraZeneca K.K. O.T., H.F., H.O. and T.O. have no conflict of interest to disclose. F.T. and A.M.L. are employees of AstraZeneca. M.A., H.K. and T.Y. are employees of AstraZeneca K.K.
Figures
References
-
- Szadkowska A, Madej A, Ziolkowska K, et al. Gender and age‐dependent effect of type 1 diabetes on obesity and altered body composition in young adults. Ann Agric Environ Med. 2015;22:124‐128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

