N-acetylcysteine prevents oxidized low-density lipoprotein-induced reduction of MG53 and enhances MG53 protective effect on bone marrow stem cells
- PMID: 31742908
- PMCID: PMC6933383
- DOI: 10.1111/jcmm.14798
N-acetylcysteine prevents oxidized low-density lipoprotein-induced reduction of MG53 and enhances MG53 protective effect on bone marrow stem cells
Abstract
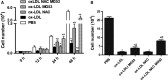
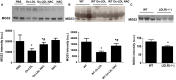
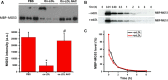
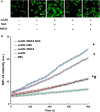
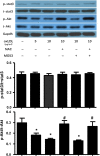
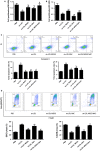
MG53 is an important membrane repair protein and partially protects bone marrow multipotent adult progenitor cells (MAPCs) against oxidized low-density lipoprotein (ox-LDL). The present study was to test the hypothesis that the limited protective effect of MG53 on MAPCs was due to ox-LDL-induced reduction of MG53. MAPCs were cultured with and without ox-LDL (0-20 μg/mL) for up to 48 hours with or without MG53 and antioxidant N-acetylcysteine (NAC). Serum MG53 level was measured in ox-LDL-treated mice with or without NAC treatment. Ox-LDL induced significant membrane damage and substantially impaired MAPC survival with selective inhibition of Akt phosphorylation. NAC treatment effectively prevented ox-LDL-induced reduction of Akt phosphorylation without protecting MAPCs against ox-LDL. While having no effect on Akt phosphorylation, MG53 significantly decreased ox-LDL-induced membrane damage and partially improved the survival, proliferation and apoptosis of MAPCs in vitro. Ox-LDL significantly decreased MG53 level in vitro and serum MG53 level in vivo without changing MG53 clearance. NAC treatment prevented ox-LDL-induced MG53 reduction both in vitro and in vivo. Combined NAC and MG53 treatment significantly improved MAPC survival against ox-LDL. These data suggested that NAC enhanced the protective effect of MG53 on MAPCs against ox-LDL through preventing ox-LDL-induced reduction of MG53.
Keywords: MG53; N-acetylcysteine; bone marrow stem cell; membrane damage; multipotent adult progenitor cells; oxidized low-density lipoprotein.
© 2019 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
None.
Figures
Similar articles
-
Cell membrane damage is involved in the impaired survival of bone marrow stem cells by oxidized low-density lipoprotein.J Cell Mol Med. 2014 Dec;18(12):2445-53. doi: 10.1111/jcmm.12424. Epub 2014 Sep 25. J Cell Mol Med. 2014. PMID: 25256620 Free PMC article.
-
Reactive oxygen species mediate oxidized low-density lipoprotein-induced inhibition of oct-4 expression and endothelial differentiation of bone marrow stem cells.Antioxid Redox Signal. 2010 Dec 15;13(12):1845-56. doi: 10.1089/ars.2010.3156. Epub 2010 Oct 12. Antioxid Redox Signal. 2010. PMID: 20836655 Free PMC article.
-
Ox-LDL modifies the behaviour of bone marrow stem cells and impairs their endothelial differentiation via inhibition of Akt phosphorylation.J Cell Mol Med. 2011 Feb;15(2):423-32. doi: 10.1111/j.1582-4934.2009.00948.x. J Cell Mol Med. 2011. PMID: 19863696 Free PMC article.
-
Hydrogen peroxide inhibits proliferation and endothelial differentiation of bone marrow stem cells partially via reactive oxygen species generation.Life Sci. 2014 Sep 1;112(1-2):33-40. doi: 10.1016/j.lfs.2014.07.016. Epub 2014 Jul 21. Life Sci. 2014. PMID: 25058920
-
Oxidized Low-Density Lipoprotein Decreases the Survival of Bone Marrow Stem Cells via Inhibition of Bcl-2 Expression.Tissue Eng Part A. 2025 Apr;31(7-8):325-333. doi: 10.1089/ten.TEA.2024.0025. Epub 2024 Jun 27. Tissue Eng Part A. 2025. PMID: 38818810
Cited by
-
MG53 attenuates nitrogen mustard-induced acute lung injury.J Cell Mol Med. 2022 Apr;26(7):1886-1895. doi: 10.1111/jcmm.16917. Epub 2022 Feb 24. J Cell Mol Med. 2022. PMID: 35199443 Free PMC article.
-
Muscle multiorgan crosstalk with MG53 as a myokine for tissue repair and regeneration.Curr Opin Pharmacol. 2021 Aug;59:26-32. doi: 10.1016/j.coph.2021.04.005. Epub 2021 May 27. Curr Opin Pharmacol. 2021. PMID: 34052525 Free PMC article. Review.
-
MG53/TRIM72: multi-organ repair protein and beyond.Front Physiol. 2024 Apr 12;15:1377025. doi: 10.3389/fphys.2024.1377025. eCollection 2024. Front Physiol. 2024. PMID: 38681139 Free PMC article. Review.
-
N-Acetylcysteine and Its Immunomodulatory Properties in Humans and Domesticated Animals.Antioxidants (Basel). 2023 Oct 16;12(10):1867. doi: 10.3390/antiox12101867. Antioxidants (Basel). 2023. PMID: 37891946 Free PMC article. Review.
-
The Pivotal Role of Mitsugumin 53 in Cardiovascular Diseases.Cardiovasc Toxicol. 2021 Jan;21(1):2-11. doi: 10.1007/s12012-020-09609-y. Epub 2020 Oct 1. Cardiovasc Toxicol. 2021. PMID: 33006052 Review.
References
-
- Yi T, Song SU. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch Pharm Res. 2012;35(2):213‐221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

