Immune responses to O-specific polysaccharide (OSP) in North American adults infected with Vibrio cholerae O1 Inaba
- PMID: 31743334
- PMCID: PMC6863522
- DOI: 10.1371/journal.pntd.0007874
Immune responses to O-specific polysaccharide (OSP) in North American adults infected with Vibrio cholerae O1 Inaba
Abstract
Background: Antibodies targeting O-specific polysaccharide (OSP) of Vibrio cholerae may protect against cholera; however, little is known about this immune response in infected immunologically naïve humans.
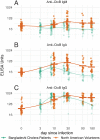
Methodology: We measured serum anti-OSP antibodies in adult North American volunteers experimentally infected with V. cholerae O1 Inaba El Tor N16961. We also measured vibriocidal and anti-cholera toxin B subunit (CtxB) antibodies and compared responses to those in matched cholera patients in Dhaka, Bangladesh, an area endemic for cholera.
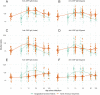
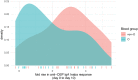
Principal findings: We found prominent anti-OSP antibody responses following initial cholera infection: these responses were largely IgM and IgA, and highest to infecting serotype with significant cross-serotype reactivity. The anti-OSP responses peaked 10 days after infection and remained elevated over baseline for ≥ 6 months, correlated with vibriocidal responses, and may have been blunted in blood group O individuals (IgA anti-OSP). We found significant differences in immune responses between naïve and endemic zone cohorts, presumably reflecting previous exposure in the latter.
Conclusions: Our results define immune responses to O-specific polysaccharide in immunologically naive humans with cholera, find that they are largely IgM and IgA, may be blunted in blood group O individuals, and differ in a number of significant ways from responses in previously humans. These differences may explain in part varying degrees of protective efficacy afforded by cholera vaccination between these two populations.
Trial registration number: ClinicalTrials.gov NCT01895855.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: Jakub K. Simon, Michael Lock, and Marc Gurwith were employed by PaxVax, Inc. Wilbur H. Chen, Caroline E. Lyon, Beth D. Kirkpatrick, Mitchell Cohen, and Myron M. Levine received research funding from PaxVax, Inc. Jakub K. Simon is currently employed by Merck & Co. All other authors report no potential conflicts.
Figures

References
-
- Hisatsune K, Kondo S, Isshiki Y, Iguchi T, Haishima Y. Occurrence of 2-O-methyl-N-(3-deoxy-L-glycero-tetronyl)-D-perosamine (4-amino-4,6-dideoxy-D-manno-pyranose) in lipopolysaccharide from Ogawa but not from Inaba O forms of O1 Vibrio cholerae. Biochem Biophys Res Commun. 1993;190(1):302–7. Epub 1993/01/15. 10.1006/bbrc.1993.1046 . - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

