Meeting Report: The Dallas Consensus Conference on Liver Transplantation for Alcohol Associated Hepatitis
- PMID: 31743578
- PMCID: PMC8151800
- DOI: 10.1002/lt.25681
Meeting Report: The Dallas Consensus Conference on Liver Transplantation for Alcohol Associated Hepatitis
Abstract
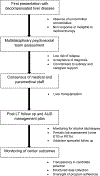
Liver transplantation (LT) for alcohol associated hepatitis (AH) remains controversial. We convened a consensus conference to examine various aspects of LT for AH. The goal was not to unequivocally endorse LT for AH; instead, it was to propose recommendations for programs that perform or plan to perform LT for AH. Criteria were established to determine candidacy for LT in the setting of AH and included the following: (1) AH patients presenting for the first time with decompensated liver disease that are nonresponders to medical therapy without severe medical or psychiatric comorbidities; (2) a fixed period of abstinence prior to transplantation is not required; and (3) assessment with a multidisciplinary psychosocial team, including a social worker and an addiction specialist/mental health professional with addiction and transplantation expertise. Supporting factors included lack of repeated unsuccessful attempts at addiction rehabilitation, lack of other substance use/dependency, acceptance of diagnosis/insight with a commitment of the patient/family to sobriety, and formalized agreement to adhere to total alcohol abstinence and counseling. LT should be avoided in AH patients who are likely to spontaneously recover. Short-term and longterm survival comparable to other indications for LT must be achieved. There should not be further disparity in LT either by indication, geography, or other sociodemographic factors. Treatment of alcohol-use disorders should be incorporated into pre- and post-LT care. The restrictive and focused evaluation process described in the initial LT experience for AH worldwide may not endure as this indication gains wider acceptance at more LT programs. Transparency in the selection process is crucial and requires the collection of objective data to assess outcomes and minimize center variation in listing. Oversight of program adherence is important to harmonize listing practices and outcomes.
Copyright © 2019 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
Figures
Comment in
-
Reply.Liver Transpl. 2020 Jul;26(7):952-953. doi: 10.1002/lt.25763. Epub 2020 May 6. Liver Transpl. 2020. PMID: 32198966 No abstract available.
-
Meeting Report: The Dallas Consensus Conference on Liver Transplantation for Alcohol Associated Hepatitis.Liver Transpl. 2020 Jul;26(7):949. doi: 10.1002/lt.25766. Liver Transpl. 2020. PMID: 32223043 No abstract available.
-
Meeting Report: The Dallas Consensus Conference on Liver Transplantation for Alcohol Associated Hepatitis.Liver Transpl. 2020 Jul;26(7):950-951. doi: 10.1002/lt.25767. Epub 2020 May 12. Liver Transpl. 2020. PMID: 32232940 No abstract available.
References
-
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009;373:2223–2233. - PubMed
-
- Im GY, Kim-Schluger L, Shenoy A, Schubert E, Goel A, Friedman SL, Florman S, et al. Early Liver Transplantation for Severe Alcoholic Hepatitis in the United States--A Single-Center Experience. Am J Transplant 2016;16:841–849. - PubMed
-
- Lee BP, Chen PH, Haugen C, Hernaez R, Gurakar A, Philosophe B, Dagher N, et al. Three-year Results of a Pilot Program in Early Liver Transplantation for Severe Alcoholic Hepatitis. Ann Surg 2017;265:20–29. - PubMed