Lysosome assembly and disassembly changes endocytosis rate through the Leishmania cell cycle
- PMID: 31743959
- PMCID: PMC7002101
- DOI: 10.1002/mbo3.969
Lysosome assembly and disassembly changes endocytosis rate through the Leishmania cell cycle
Abstract
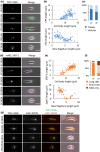
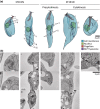
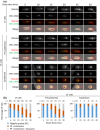
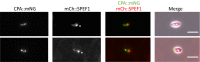
The Leishmania lysosome has an atypical structure, consisting of an elongated vesicle-filled tubule running along the anterior-posterior axis of the cell, which is termed the multivesicular tubule (MVT) lysosome. Alongside, the MVT lysosome is one or more microtubules, the lysosomal microtubule(s). Previous work indicated there were cell cycle-related changes in MVT lysosome organization; however, these only provided snapshots and did not connect the changes in the lysosomal microtubule(s) or lysosomal function. Using mNeonGreen tagged cysteine peptidase A and SPEF1 as markers of the MVT lysosome and lysosomal microtubule(s), we examined the dynamics of these structures through the cell cycle. Both the MVT lysosome and lysosomal microtubule(s) elongated at the beginning of the cell cycle before plateauing and then disassembling in late G2 before cytokinesis. Moreover, the endocytic rate in cells where the MVT lysosome and lysosomal microtubule(s) had disassembled was extremely low. The dynamic nature of the MVT lysosome and lysosomal microtubule(s) parallels that of the Trypanosoma cruzi cytostome/cytopharynx, which also has a similar membrane tubule structure with associated microtubules. As the cytostome/cytopharynx is an ancestral feature of the kinetoplastids, this suggests that the Leishmania MVT lysosome and lysosomal microtubule(s) are a reduced cytostome/cytopharynx-like feature.
Keywords: Leishmania; cell cycle; endocytosis; lysosome.
© 2019 The Authors. MicrobiologyOpen published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
The cytostome-cytopharynx complex of Trypanosoma cruzi epimastigotes disassembles during cell division.J Cell Sci. 2017 Jan 1;130(1):164-176. doi: 10.1242/jcs.187419. Epub 2016 Jun 30. J Cell Sci. 2017. PMID: 27363990
-
The three-dimensional structure of the cytostome-cytopharynx complex of Trypanosoma cruzi epimastigotes.J Cell Sci. 2014 May 15;127(Pt 10):2227-37. doi: 10.1242/jcs.135491. Epub 2014 Mar 7. J Cell Sci. 2014. PMID: 24610945
-
Loss of the cytostome-cytopharynx and endocytic ability are late events in Trypanosoma cruzi metacyclogenesis.J Struct Biol. 2016 Dec;196(3):319-328. doi: 10.1016/j.jsb.2016.07.018. Epub 2016 Jul 30. J Struct Biol. 2016. PMID: 27480509
-
Developmental changes in lysosome morphology and function Leishmania parasites.Int J Parasitol. 2002 Nov;32(12):1435-45. doi: 10.1016/s0020-7519(02)00140-6. Int J Parasitol. 2002. PMID: 12392909 Review.
-
Cell biology of host-parasite membrane interactions in leishmaniasis.Ciba Found Symp. 1983;99:113-37. doi: 10.1002/9780470720806.ch7. Ciba Found Symp. 1983. PMID: 6357669 Review.
Cited by
-
Tubulin detyrosination shapes Leishmania cytoskeletal architecture and virulence.Proc Natl Acad Sci U S A. 2025 Jan 21;122(3):e2415296122. doi: 10.1073/pnas.2415296122. Epub 2025 Jan 14. Proc Natl Acad Sci U S A. 2025. PMID: 39808657 Free PMC article.
-
The single flagellum of Leishmania has a fixed polarisation of its asymmetric beat.J Cell Sci. 2020 Oct 22;133(20):jcs246637. doi: 10.1242/jcs.246637. J Cell Sci. 2020. PMID: 33093230 Free PMC article.
-
Basal Body Protein TbSAF1 Is Required for Microtubule Quartet Anchorage to the Basal Bodies in Trypanosoma brucei.mBio. 2020 Jun 9;11(3):e00668-20. doi: 10.1128/mBio.00668-20. mBio. 2020. PMID: 32518185 Free PMC article.
-
Whole cell reconstructions of Leishmania mexicana through the cell cycle.PLoS Pathog. 2024 Feb 28;20(2):e1012054. doi: 10.1371/journal.ppat.1012054. eCollection 2024 Feb. PLoS Pathog. 2024. PMID: 38416776 Free PMC article.
-
The Leishmania donovani LDBPK_220120.1 Gene Encodes for an Atypical Dual Specificity Lipid-Like Phosphatase Expressed in Promastigotes and Amastigotes; Substrate Specificity, Intracellular Localizations, and Putative Role(s).Front Cell Infect Microbiol. 2021 Mar 25;11:591868. doi: 10.3389/fcimb.2021.591868. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33842381 Free PMC article.
References
-
- Alcantara, C. L. , Vidal, J. C. , de Souza, W. , & Cunha‐e‐Silva, N. L. (2014). The three‐dimensional structure of the cytostome‐cytopharynx complex of Trypanosoma cruzi epimastigotes. Journal of Cell Science, 127, 2227–2237. - PubMed
-
- Alcantara, C. L. , Vidal, J. C. , de Souza, W. , & Cunha‐E‐Silva, N. L. (2017). The cytostome–cytopharynx complex of Trypanosoma cruzi epimastigotes disassembles during cell division. Journal of Cell Science, 130, 164–176. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

