Reversal of Olfactory Disturbance in Allergic Rhinitis Related to OMP Suppression by Intranasal Budesonide Treatment
- PMID: 31743968
- PMCID: PMC6875474
- DOI: 10.4168/aair.2020.12.1.110
Reversal of Olfactory Disturbance in Allergic Rhinitis Related to OMP Suppression by Intranasal Budesonide Treatment
Abstract
Purpose: We evaluated the severity of olfactory disturbance (OD) in the murine model of allergic rhinitis (AR) and local allergic rhinitis (LAR) in mice. We also investigated the therapeutic effect of an intranasal steroid on OD.
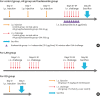
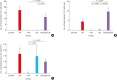
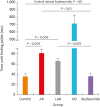
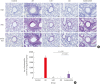
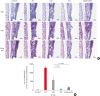
Methods: Forty BALB/c mice were divided into 5 groups (n = 8 for each). The control group was sensitized intraperitoneally (i.p.) and challenged intranasally (i.n.) with saline. Mice in the AR group got i.p. and i.n. ovalbumin (OVA) administration for AR induction. The LAR group was challenged i.n. with 1% OVA for inducing local nasal allergic inflammation, without inducing the systemic allergy. The OD group got an i.p. methimazole administration (75 mg/kg) to induce total destruction of olfactory mucosa. Mice in the intranasal budesonide group received i.n. budesonide (12.8 μ per time, 30 minutes after the i.n. OVA challenge) while using OVA to cause systemic allergies. We conducted a buried-food pellet test to functionally assess the degree of OD in each group by measuring the time taken until finding hidden food. We evaluated the damage to olfactory epithelium using histopathologic evaluation and compared the degree of olfactory marker protein (OMP) expression in olfactory epithelium using immunofluorescent staining.
Results: Mice of the AR (81.3 ± 19.8 seconds) and LAR groups (66.2 ± 12.7 seconds) spent significantly more time to detect the pellets than the control group (35.6 ± 12.2 seconds, P < 0.01). After treatment, the intranasal budesonide group exhibited significantly better results (35.8 ± 11.9 seconds) compared with the AR and LAR groups (P < 0.01). The AR and LAR groups showed considerable olfactory epithelial damage and suppression of OMP expression compared with the control group. In the intranasal budesonide group, the olfactory lesions and OMP expression had improved substantially.
Conclusions: OD may be caused by olfactory epithelial damage and suppression of OMP expression in nasal allergic inflammation and could be reversed using an intranasal steroid.
Keywords: Olfactory mucosa; allergic rhinitis; odorants; olfaction disorders; olfactory marker protein; ovalbumin; quality of life; steroids.
Copyright © 2020 The Korean Academy of Asthma, Allergy and Clinical Immunology · The Korean Academy of Pediatric Allergy and Respiratory Disease.
Conflict of interest statement
There are no financial interests or other issues that might lead to conflict of interest.
Figures



References
-
- Brożek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140:950–958. - PubMed
-
- Cowart BJ, Flynn-Rodden K, McGeady SJ, Lowry LD. Hyposmia in allergic rhinitis. J Allergy Clin Immunol. 1993;91:747–751. - PubMed
-
- Binder E, Holopainen E, Malmberg H, Salo O. Anamnestic data in allergic rhinitis. Allergy. 1982;37:389–396. - PubMed
-
- Chaiyasate S, Roongrotwattanasiri K, Fooanant S, Sumitsawan Y. Key nasal symptoms predicting a positive skin test in allergic rhinitis and patient characteristics according to ARIA classification. J Med Assoc Thai. 2009;92:377–381. - PubMed
-
- Di Lorenzo G, Pacor ML, Amodio E, Leto-Barone MS, La Piana S, D'Alcamo A, et al. Differences and similarities between allergic and nonallergic rhinitis in a large sample of adult patients with rhinitis symptoms. Int Arch Allergy Immunol. 2011;155:263–270. - PubMed

