Perfluorooctanoate in Aqueous Urea Solutions: Micelle Formation, Structure, and Microenvironment
- PMID: 31744078
- PMCID: PMC6888096
- DOI: 10.3390/ijms20225761
Perfluorooctanoate in Aqueous Urea Solutions: Micelle Formation, Structure, and Microenvironment
Abstract
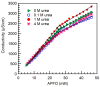
Fluorinated surfactants are used in a wide range of applications that involve aqueous solvents incorporating various additives. The presence of organic compounds such as urea is expected to affect the self-assembly of fluorinated surfactants, however, very little is known about this. We investigated the effect of urea on the micellization in water of the common fluorinated surfactant ammonium perfluorooctanoate (APFO), and on the structure and microenvironment of the micelles that APFO forms. Addition of urea to aqueous APFO solution decreased the critical micellization concentration (CMC) and increased the counterion dissociation. The observed increase in surface area per APFO headgroup and decrease in packing density at the micelle surface suggest the localization of urea at the micelle surface in a manner that reduces headgroup repulsions. Micropolarity data further support this picture. The results presented here indicate that significant differences exist between urea effects on fluorinated surfactant and on hydrocarbon surfactant micellization in aqueous solution. For example, the CMC of sodium dodecyl sulfate (SDS) increased with urea addition, while the increase in surface area per headgroup and packing density of SDS with urea addition are much lower than those observed for APFO. This study informs fluorinated surfactant fate and transport in the environment, and also applications involving aqueous media in which urea or similar additives are present.
Keywords: denaturation; perfluoroalkyl substances (PFAS); surfactant; urea; water.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Wallqvist A., Covell D.G., Thirumalai D. Hydrophobic interactions in aqueous urea solutions with implications for the mechanism of protein denaturation. J. Am. Chem. Soc. 1998;120:427–428. doi: 10.1021/ja972053v. - DOI
-
- Alexandridis P., Ghasemi M., Furlani E.P., Tsianou M. Solvent processing of cellulose for effective bioresource utilization. Curr. Opin. Green Sustain. Chem. 2018;14:40–52. doi: 10.1016/j.cogsc.2018.05.008. - DOI
-
- Walrafen G.E. Raman spectral studies of the effects of urea and sucrose on water structure. J. Chem. Phys. 1966;44:3726–3727. doi: 10.1063/1.1726526. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

