Pyrido[2,3- d]pyrimidin-7(8 H)-ones: Synthesis and Biomedical Applications
- PMID: 31744155
- PMCID: PMC6891647
- DOI: 10.3390/molecules24224161
Pyrido[2,3- d]pyrimidin-7(8 H)-ones: Synthesis and Biomedical Applications
Abstract
Pyrido[2,3-d]pyrimidines (1) are a type of privileged heterocyclic scaffolds capable of providing ligands for several receptors in the body. Among such structures, our group and others have been particularly interested in pyrido[2,3-d]pyrimidine-7(8H)-ones (2) due to the similitude with nitrogen bases present in DNA and RNA. Currently there are more than 20,000 structures 2 described which correspond to around 2900 references (half of them being patents). Furthermore, the number of references containing compounds of general structure 2 have increased almost exponentially in the last 10 years. The present review covers the synthetic methods used for the synthesis of pyrido[2,3-d]pyrimidine-7(8H)-ones (2), both starting from a preformed pyrimidine ring or a pyridine ring, and the biomedical applications of such compounds.
Keywords: 5,6-dihydropyrido[2,3-d]pyrimidin-7(8H)-ones; biological activity; pyrido[2,3-d]pyrimidines; substitution pattern.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

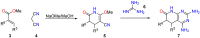




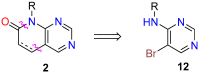


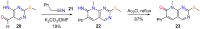








References
-
- Sako M. Product class 19: Pyridopyrimidines. Sci. Synth. 2004;16:1155–1267.
-
- Akssira M., Guillaumet G., Routier S. Recent advances in the chemistry and biology of pyridopyrimidines. Eur. J. Med. Chem. 2015;95:76–95. - PubMed
-
- Evans B.E., Rittle K.E., Bock M.G., DiPardo R.M., Freidinger R.M., Whitter W.L., Lundell G.F., Veber D.F., Anderson P.S., Chang R.S. Methods for drug discovery: Development of potent, selective, orally effective cholecystokinin antagonists. J. Med. Chem. 1988;31:2235–2246. doi: 10.1021/jm00120a002. - DOI - PubMed
-
- Altomare C.D. Privileged heterocyclic scaffolds in chemical biology and drug discovery: Synthesis and bioactivity. Chem. Heterocycl. Compd. 2017;53:239.
-
- Theivendren P.S., Caiado R.J., Phadte V.D., Silveira K.V. A mini review of pyrimidine and fused pyrimidine marketed drugs. Res. Pharm. 2012;2:1–9.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

