Spirocyclic Motifs in Natural Products
- PMID: 31744211
- PMCID: PMC6891393
- DOI: 10.3390/molecules24224165
Spirocyclic Motifs in Natural Products
Abstract
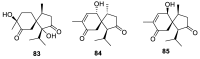
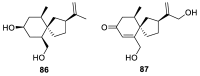
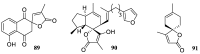
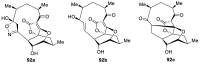
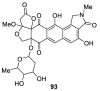
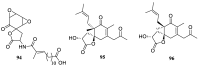
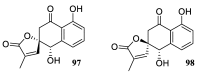
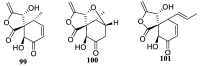
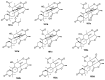
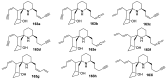
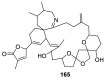
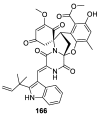
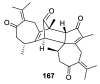
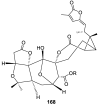
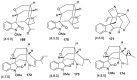
Spirocyclic motifs are emerging privileged structures for drug discovery. They are also omnipresent in the natural products domain. However, until today, no attempt to analyze the structural diversity of various spirocyclic motifs occurring in natural products and their relative populations with unique compounds reported in the literature has been undertaken. This review aims to fill that void and analyze the diversity of structurally unique natural products containing spirocyclic moieties of various sizes.
Keywords: biological activity; chemical diversity; natural products; privileged structures; spirocycles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








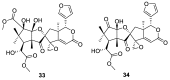
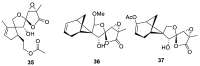

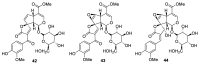
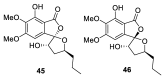











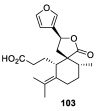
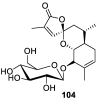

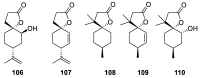

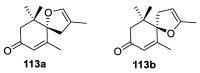






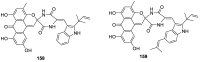


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

