Restriction of intracellular Salmonella replication by restoring TFEB-mediated xenophagy
- PMID: 31744366
- PMCID: PMC8386624
- DOI: 10.1080/15548627.2019.1689770
Restriction of intracellular Salmonella replication by restoring TFEB-mediated xenophagy
Abstract
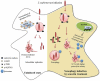
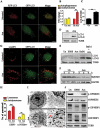
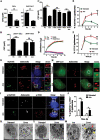
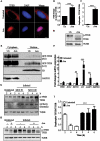
Macroautophagy/autophagy functions as a part of the innate immune system in clearing intracellular pathogens. Although this process is well known, the mechanisms that control antibacterial autophagy are not clear. In this study we show that during intracellular Salmonella typhimurium infection, the activity of TFEB (transcription factor EB), a master regulator of autophagy and lysosome biogenesis, is suppressed by maintaining it in a phosphorylated state on the lysosomes. Furthermore, we have identified a novel, antibacterial small molecule autophagy (xenophagy) modulator, acacetin. The xenophagy effect exerted by acacetin occurs in an MTOR (mechanistic target of rapamycin kinase)-independent, TFEB-dependent manner. Acacetin treatment results in persistently maintaining active TFEB in the nucleus and also in TFEB mediated induction of functional lysosomes that target Salmonella-containing vacuoles (SCVs). The enhanced proteolytic activity due to deployment of lysosomes results in clamping down Salmonella replication in SCVs. Acacetin is effective as a xenophagy compound in an in vivo mouse model of infection and reduces intracellular Salmonella burden.
Abbreviations: 3-MA: 3-methyladenine; BafA1: bafilomycin A1; CFU: colony-forming units; DQ-BSA: dye quenched-bovine serum albumin; EEA1: early endosome antigen 1; FITC: fluorescein isothiocyanate; FM 4-64: pyridinium,4-(6-[4-{diethylamino}phenyl]-1,3,5-hexatrienyl)-1-(3[triethylammonio] propyl)-dibromide; GFP: green fluorescent protein; LAMP1: lysosomal associated membrane protein 1; MAPILC3/LC3: microtubule associated protein 1 light chain 3; MOI: multiplicity of infection; MTOR: mechanistic target of rapamycin kinase; RFP: red fluorescent protein; SCVs: Salmonella-containing vacuoles; SD: standard deviation; SDS: sodium dodecyl sulfate; SEM: standard mean error; SQSTM1: sequestosome 1; TBK1: TANK binding kinase 1; TFEB: transcription factor EB.
Keywords: Salmonella typhimurium; Salmonella-containing vacuoles; Acacetin; MTOR-independent; TFEB; autolysosomes; lysosomes; xenophagy.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures
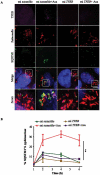
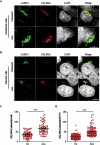
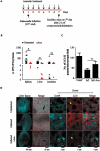
References
-
- Rich KA, Burkett C, Webster P.. Cytoplasmic bacteria can be targets for autophagy. Cell Microbiol. 2003;5:455–468. - PubMed
-
- Gutierrez MG, Master SS, Singh SB, et al. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. - PubMed
-
- Nakagawa I, Amano A, Mizushima N, et al. Autophagy defends cells against invading group A Streptococcus. Science. 2004;306:1037–1040. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
