Analyzing the value of an educational program for psoriasis patients: a prospective controlled pilot study
- PMID: 31744488
- PMCID: PMC6862860
- DOI: 10.1186/s12889-019-7778-x
Analyzing the value of an educational program for psoriasis patients: a prospective controlled pilot study
Abstract
Background: Psoriasis is a chronic inflammatory skin disease associated with a reduced life-quality. Severe disease forms put the patients at risk for life-treating cardiovascular events, metabolic, and other immune-mediated disorders. Psoriasis patients are often not sufficiently informed about their condition leading to suboptimal treatment adherence and, consequently, worse patient outcome. We investigated the value of an educational program on knowledge and self-expertise about the disease in psoriasis patients in general and dependent on age and disease duration.
Methods: Regular visit psoriasis-patients were asked to participate and choose to receive an additional educational program or not. Participating patients (n = 53) filled out two questionnaires: one at study inclusion and one at the next regular visit or after the absolved educational program. Surveys included disease knowledge assessment and numeric rating scales (0-10) for self-expertise about the disease, therapy adherence, and therapy satisfaction. The Dermatology Life Quality Index (DLQI) was used to investigate the quality of life. All continuous parameters were examined for statistically significant differences by paired t-test or unpaired t-test. Continuous parameters without Gaussian distribution were analyzed with the Wilcoxon matched pairs test or the Mann-Whitney test. For all categorical parameters, Fisher's exact test was used.
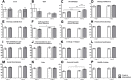
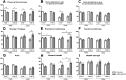
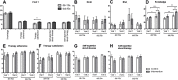
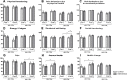
Results: Patients who chose to be educated (n = 24) showed a significant increase in knowledge, self-expertise about the disease and amelioration of general health. No positive short-term effects were seen on the quality of life and therapy adherence. Analyzing the effect of age and disease duration, the educational program led to significant improvement of the emotional well-being in older patients (≥50 years) and with a longer disease duration as well as significant amelioration of the self-expertise about psoriasis in younger patients (< 50 years).
Conclusions: Patients who chose to participate in an educational program show a higher gain in knowledge and self-expertise about the psoriatic disease. Educational program thus might have a positive effect on the long-term management of psoriasis. Further long-term studies are needed to provide evidence for the influence educational programs have on outcome, quality of life, and treatment adherence of psoriatic patients.
Trial registration: Deutsches Register Klinischer Studien DRKS00017318 (09.10.2019), retrospectively registered.
Keywords: Adherence knowledge about disease; Educational program; Patient education; Psoriasis; Self-expertise about disease.
Conflict of interest statement
Prof. Peitsch served as investigator for AbbVie, Boehringer Ingelheim, Eli Lilly, Janssen-Cilag, Merck, Novartis, Pfizer and UCB Pharma; participated in a clinical trial supported by Array Biopharma and MSD; was member of advisory boards of Eli Lilly, LEO Pharma, MSD, Novartis, Pfizer and UCB Pharma; obtained honoraria from ALK-Abello, AbbVie, Biotest, BMS, Janssen-Cilag, MSD, Novartis, Pfizer, Dr. Pfleger GmbH and Roche; and received support for conferences from AbbVie, Actelion, ALK-Abello, Alma Lasers, Almirall, ARC Lasers, Asclepion, BMS, Celgene, Dermapharm, Dermasence, Galderma, GSK, Janssen-Cilag, L’Oreal, La Roche Posay, LEO Pharma, Medac, MSD, Novartis, Pierre Fabre, P&M Cosmetics, Pfizer and Roche. Dr. Schaarschmidt conducted clinical trials for Novartis, Janssen-Cilag, Merck, LEO Pharma, Abbvie, and Celgene and received financial support for participation in conferences from Abbvie, ALK-Abello, Biogen Inc. and MSD. Dr. Schmieder conducted clinical trials for Novartis, Janssen-Cilag, Merck, LEO Pharma, Boehringer-Ingelheim, Abbvie, Eli Lilly, Celgene and Pfizer, received honoraria from Novartis and Janssen-Cilag and obtained support for conferences from Abbvie, Novartis, Janssen-Cilag and Pfizer. Mrs. Corinna Bubak and Mrs. Lisa Schöben have no conflict of interest to declare. The study presented here was not supported by pharmaceutical companies, and the conflicts of interest have no impact on its content.
Figures

Similar articles
-
Educational and motivational support service: a pilot study for mobile-phone-based interventions in patients with psoriasis.Br J Dermatol. 2013 Jan;168(1):201-5. doi: 10.1111/j.1365-2133.2012.11205.x. Epub 2012 Dec 13. Br J Dermatol. 2013. PMID: 23240729 Clinical Trial.
-
[Therapeutic benefit of a registered psychoeducation program on treatment adherence, objective and subjective quality of life: French pilot study for schizophrenia].Encephale. 2017 May;43(3):235-240. doi: 10.1016/j.encep.2015.12.028. Epub 2016 Sep 19. Encephale. 2017. PMID: 27658989 French.
-
Acceptability and feasibility of a 12-week yoga vs. educational film program for the management of restless legs syndrome (RLS): study protocol for a randomized controlled trial.Trials. 2019 Feb 15;20(1):134. doi: 10.1186/s13063-019-3217-7. Trials. 2019. PMID: 30770767 Free PMC article.
-
Educational interventions to improve quality of life in people with chronic inflammatory skin diseases: systematic reviews of clinical effectiveness and cost-effectiveness.Health Technol Assess. 2015 Oct;19(86):1-176, v-vi. doi: 10.3310/hta19860. Health Technol Assess. 2015. PMID: 26502807 Free PMC article. Review.
-
E-health: Web-guided therapy and disease self-management in ulcerative colitis. Impact on disease outcome, quality of life and compliance.Dan Med J. 2012 Jul;59(7):B4478. Dan Med J. 2012. PMID: 22759851 Review.
Cited by
-
Patient Education with New Media Integration Self-Management Support Model Improves Therapeutic Outcomes of Rosacea Patients.Patient Prefer Adherence. 2023 Sep 27;17:2395-2400. doi: 10.2147/PPA.S431955. eCollection 2023. Patient Prefer Adherence. 2023. PMID: 37790861 Free PMC article.
-
Impact of an eHealth Smartphone App on the Mental Health of Patients With Psoriasis: Prospective Randomized Controlled Intervention Study.JMIR Mhealth Uhealth. 2021 Oct 25;9(10):e28149. doi: 10.2196/28149. JMIR Mhealth Uhealth. 2021. PMID: 34431478 Free PMC article. Clinical Trial.
-
Evaluation of knowledge in the field of proper skin care and exacerbating factors in patients with psoriasis.Postepy Dermatol Alergol. 2022 Apr;39(2):401-406. doi: 10.5114/ada.2021.107099. Epub 2021 Jun 20. Postepy Dermatol Alergol. 2022. PMID: 35645668 Free PMC article.
-
Cinematic rendering in rheumatic diseases-Photorealistic depiction of pathologies improves disease understanding for patients.Front Med (Lausanne). 2022 Aug 3;9:946106. doi: 10.3389/fmed.2022.946106. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35991672 Free PMC article.
-
Non-pharmacological interventions for patients with psoriasis: a scoping review.BMJ Open. 2023 Nov 24;13(11):e074752. doi: 10.1136/bmjopen-2023-074752. BMJ Open. 2023. PMID: 38000814 Free PMC article.
References
-
- Boehncke WH, Schon MP. Psoriasis. Lancet (London Engl) 2015;386(9997):983–994. - PubMed
-
- Nast A, Amelunxen L, Augustin M, Boehncke WH, Dressler C, Gaskins M, et al. S3 guideline for the treatment of psoriasis vulgaris, update - short version part 2 - special patient populations and treatment situations. Journal der Deutschen Dermatologischen Gesellschaft. J German Soc Dermatol. 2018;16(6):806–813. - PubMed
-
- Bohm D, Stock Gissendanner S, Bangemann K, Snitjer I, Werfel T, Weyergraf A, et al. Perceived relationships between severity of psoriasis symptoms, gender, stigmatization and quality of life. J Eur Acad Dermatol Venereol. 2013;27(2):220–226. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

