Separation of 9-Fluorenylmethyloxycarbonyl Amino Acid Derivatives in Micellar Systems of High-Performance Thin-Layer Chromatography and Pressurized Planar Electrochromatography
- PMID: 31745145
- PMCID: PMC6864085
- DOI: 10.1038/s41598-019-53468-9
Separation of 9-Fluorenylmethyloxycarbonyl Amino Acid Derivatives in Micellar Systems of High-Performance Thin-Layer Chromatography and Pressurized Planar Electrochromatography
Abstract
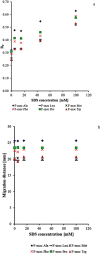
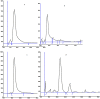
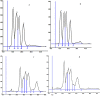
The problems with separation of amino acid mixtures in reversed-phase mode are the result of their hydrophilic nature. The derivatisation of the amino group of mentioned above solutes leads to their solution. For this purpose, 9-fluorenylmethoxycarbonyl chloroformate (f-moc-Cl) as the derivatisation reagent is often used. In our study, the separation of some f-moc- amino acid derivatives (alanine, phenylalanine, leucine, methionine, proline and tryptophan) with the use of micellar systems of reversed-phase high-performance thin-layer chromatography (HPTLC) and pressurized planar electrochromatography (PPEC) is investigated. The effect of surfactant concentration, its type (anionic, cationic and non-ionic) and mobile phase buffer pH on the discussed above solute migration distances are presented. Our work reveals that the increase of sodium dodecylsulphate concentration in the mobile phase has a different effect on solute retention in HPTLC and PPEC. Moreover, it also affects the order of solutes in both techniques. In PPEC, in contrast to the HPTLC technique, the mobile phase pH affects solute retention. The type of surfactant in the mobile phase also impacts solute retention and migration distances. A mobile phase containing SDS improves system efficiency in both techniques. Herein, such an effect is presented for the first time.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Separation of some vitamins in reversed-phase thin-layer chromatography and pressurized planar electrochromatography with eluent containing surfactant.Sci Rep. 2021 Nov 8;11(1):21851. doi: 10.1038/s41598-021-01323-1. Sci Rep. 2021. PMID: 34750458 Free PMC article.
-
Comparison of Phenolic Compound Separations by HPTLC and PPEC with SDS as the Mobile Phase Component.J Anal Methods Chem. 2019 Jan 10;2019:6845340. doi: 10.1155/2019/6845340. eCollection 2019. J Anal Methods Chem. 2019. PMID: 30733887 Free PMC article.
-
The influence of pH on retention and migration of peptides in systems with octadecyl silica-based adsorbent by high-performance thin-layer chromatography and pressurized planar electrochromatography techniques.J Chromatogr A. 2018 Jan 26;1534:179-187. doi: 10.1016/j.chroma.2017.12.063. Epub 2017 Dec 26. J Chromatogr A. 2018. PMID: 29317103
-
Pressurized planar electrochromatography.J Chromatogr A. 2011 May 13;1218(19):2636-47. doi: 10.1016/j.chroma.2011.03.014. Epub 2011 Mar 17. J Chromatogr A. 2011. PMID: 21481884 Review.
-
Planar electrochromatography.J Chromatogr A. 2004 Jul 30;1044(1-2):83-96. doi: 10.1016/j.chroma.2004.04.055. J Chromatogr A. 2004. PMID: 15354430 Review.
Cited by
-
Separation of some vitamins in reversed-phase thin-layer chromatography and pressurized planar electrochromatography with eluent containing surfactant.Sci Rep. 2021 Nov 8;11(1):21851. doi: 10.1038/s41598-021-01323-1. Sci Rep. 2021. PMID: 34750458 Free PMC article.
-
High performance thin layer chromatography with eluents containing SDS aided by UV- and Raman spectroscopy.Sci Rep. 2025 Apr 28;15(1):14777. doi: 10.1038/s41598-025-99617-1. Sci Rep. 2025. PMID: 40295698 Free PMC article.
References
-
- Shin S, et al. Simultaneous analysis of acetylcarnitine, proline, hydroxyproline, citrulline, and arginine as potential plasma biomarkers to evaluate NSAIDs-induced gastric injury by liquid chromatography–tandem mass spectrometry. J Pharm Biomed Anal. 2019;165:101–111. doi: 10.1016/j.jpba.2018.11.051. - DOI - PubMed
LinkOut - more resources
Full Text Sources

