Next-Generation Sequencing Reveals Novel Genetic Variants (SRY, DMRT1, NR5A1, DHH, DHX37) in Adults With 46,XY DSD
- PMID: 31745530
- PMCID: PMC6855215
- DOI: 10.1210/js.2019-00306
Next-Generation Sequencing Reveals Novel Genetic Variants (SRY, DMRT1, NR5A1, DHH, DHX37) in Adults With 46,XY DSD
Abstract
Context: The genetic basis of human sex development is slowly being elucidated, and >40 different genetic causes of differences (or disorders) of sex development (DSDs) have now been reported. However, reaching a specific diagnosis using traditional approaches can be difficult, especially in adults where limited biochemical data may be available.
Objective: We used a targeted next-generation sequencing approach to analyze known and candidate genes for DSDs in individuals with no specific molecular diagnosis.
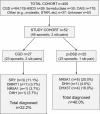
Participants and design: We studied 52 adult 46,XY women attending a single-center adult service, who were part of a larger cohort of 400 individuals. Classic conditions such as17β-hydroxysteroid dehydrogenase deficiency type 3, 5α-reductase deficiency type 2, and androgen insensitivity syndrome were excluded. The study cohort had broad working diagnoses of complete gonadal dysgenesis (CGD) (n = 27) and partially virilized 46,XY DSD (pvDSD) (n = 25), a group that included partial gonadal dysgenesis and those with a broad "partial androgen insensitivity syndrome" label. Targeted sequencing of 180 genes was undertaken.
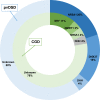
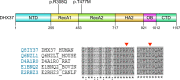
Results: Overall, a likely genetic cause was found in 16 of 52 (30.8%) individuals (22.2% CGD, 40.0% pvDSD). Pathogenic variants were found in sex-determining region Y (SRY; n = 3), doublesex and mab-3-related transcription factor 1 (DMRT1; n = 1), NR5A1/steroidogenic factor-1 (SF-1) (n = 1), and desert hedgehog (DHH; n = 1) in the CGD group, and in NR5A1 (n = 5), DHH (n = 1), and DEAH-box helicase 37 (DHX37; n = 4) in the pvDSD group.
Conclusions: Reaching a specific diagnosis can have clinical implications and provides insight into the role of these proteins in sex development. Next-generation sequencing approaches are invaluable, especially in adult populations or where diagnostic biochemistry is not possible.
Keywords: DHX37; DSD; SRY; desert hedgehog; sex determination; steroidogenic factor-1.
Copyright © 2019 Endocrine Society.
Figures




References
-
- Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346(6281):240–244. - PubMed
-
- Berta P, Hawkins JR, Sinclair AH, Taylor A, Griffiths BL, Goodfellow PN, Fellous M. Genetic evidence equating SRY and the testis-determining factor. Nature. 1990;348(6300):448–450. - PubMed
-
- Koopman P, Münsterberg A, Capel B, Vivian N, Lovell-Badge R. Expression of a candidate sex-determining gene during mouse testis differentiation. Nature. 1990;348(6300):450–452. - PubMed
-
- Audí L, Ahmed SF, Krone N, Cools M, McElreavey K, Holterhus PM, Greenfield A, Bashamboo A, Hiort O, Wudy SA, McGowan R; The EU COST Action. Genetics in endocrinology: approaches to molecular genetic diagnosis in the management of differences/disorders of sex development (DSD): position paper of EU COST Action BM 1303 “DSDnet”. Eur J Endocrinol. 2018;179(4):R197–R206. - PubMed
-
- Phelan N, Williams EL, Cardamone S, Lee M, Creighton SM, Rumsby G, Conway GS. Screening for mutations in 17β-hydroxysteroid dehydrogenase and androgen receptor in women presenting with partially virilised 46,XY disorders of sex development. Eur J Endocrinol. 2015;172(6):745–751. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
