Knockdown of Methoprene-Tolerant Arrests Ovarian Development in the Sogatella furcifera (Hemiptera: Delphacidae)
- PMID: 31745557
- PMCID: PMC6864119
- DOI: 10.1093/jisesa/iez113
Knockdown of Methoprene-Tolerant Arrests Ovarian Development in the Sogatella furcifera (Hemiptera: Delphacidae)
Abstract
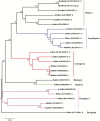
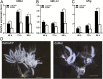
Juvenile hormone (JH) is responsible for repressing larval metamorphosis and inducing vitellogenesis and egg production in insects. Methoprene-tolerant (Met) is known to be an intracellular receptor and transducer of JH. We examined the role of Met in ovarian development in the rice pest Sogatella furcifera (Horváth). We first cloned and sequenced S. furcifera Met (SfMet). The SfMet protein belongs to the basic helix-loop-helix/Per-Arnt-Sim (bHLH-PAS) family with a bHLH domain and two PAS domains (PAS-A and PAS-B). SfMet was expressed in all developmental stages and tissues but was most highly expressed in the ovaries of adult females. Furthermore, RNA interference (RNAi) mediated silencing of SfMet substantially reduced the expression of SfVg, decreased yolk protein deposition and blocked oocyte maturation and ovarian development. These results demonstrate that SfMet plays a key role in female reproduction in S. furcifera and suggest that targeting this gene could be an effective way of controlling this pest.
Keywords: Sogatella furcifera; juvenile hormone; methoprene-tolerant; vitellogenin.
© The Author(s) 2019. Published by Oxford University Press on behalf of Entomological Society of America.
Figures


Similar articles
-
Molecular characterization of the Krüppel-homolog 1 and its role in ovarian development in Sogatella furcifera (Hemiptera: Delphacidae).Mol Biol Rep. 2020 Feb;47(2):1099-1106. doi: 10.1007/s11033-019-05206-7. Epub 2019 Nov 29. Mol Biol Rep. 2020. PMID: 31784857
-
Hairy and Krüppel homolog 1 Comediate the Action of Juvenile Hormone/Methoprene-Tolerant Signaling Pathway in Vitellogenesis of Spodoptera frugiperda (J.E. Smith).J Agric Food Chem. 2025 Jan 15;73(2):1122-1130. doi: 10.1021/acs.jafc.4c08653. Epub 2025 Jan 2. J Agric Food Chem. 2025. PMID: 39745858
-
Juvenile Hormone receptor Met is essential for ovarian maturation in the Desert Locust, Schistocerca gregaria.Sci Rep. 2019 Jul 25;9(1):10797. doi: 10.1038/s41598-019-47253-x. Sci Rep. 2019. PMID: 31346226 Free PMC article.
-
The juvenile hormone signaling pathway in insect development.Annu Rev Entomol. 2013;58:181-204. doi: 10.1146/annurev-ento-120811-153700. Epub 2012 Sep 17. Annu Rev Entomol. 2013. PMID: 22994547 Review.
-
Molecular Mechanisms of Transcription Activation by Juvenile Hormone: A Critical Role for bHLH-PAS and Nuclear Receptor Proteins.Insects. 2012 Mar 22;3(1):324-38. doi: 10.3390/insects3010324. Insects. 2012. PMID: 26467963 Free PMC article. Review.
Cited by
-
Molecular characterization of Vitellogenin-like1 gene in Sogatella furcifera (Hemiptera: Delphacidae), and its function on reproduction.J Insect Sci. 2024 Jan 1;24(1):17. doi: 10.1093/jisesa/ieae013. J Insect Sci. 2024. PMID: 38412292 Free PMC article.
-
Risk Assessment of RNAi-Based Potential Pesticide dsNlAtg3 and Its Homologues for Nilaparvata lugens and Non-Target Organisms.Insects. 2025 Feb 19;16(2):225. doi: 10.3390/insects16020225. Insects. 2025. PMID: 40003854 Free PMC article.
-
Cloning and functional analysis of the juvenile hormone receptor gene CsMet in Coccinella septempunctata.J Insect Sci. 2024 Jul 1;24(4):2. doi: 10.1093/jisesa/ieae065. J Insect Sci. 2024. PMID: 38958929 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

